Modern medicine is challenged by curing genetic diseases. New hope for patients and their families has been brought by developments in genetics research, but the safety of these methods is still of concern.
A team of biologists at the University of California San Diego describe a new approach that may correct genetic defects in the future. The foundation for novel gene therapy strategies is provided by their strategy, which uses natural DNA repair machinery.
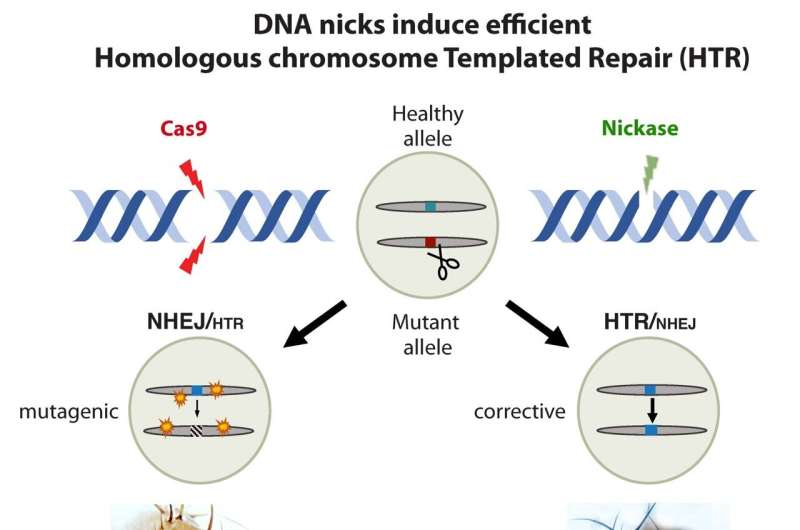
The two copies of genes that parents pass on to their children can be different from one another. A functional sequence counterpart will be found on the other side of the chromosomes when there is a genetic change. This fact was exploited by the researchers with the use of genetic editing tools.
According to Guichard, the senior author of the study, the healthy variant can be used by the cell's repair machinery to correct the defect.
Fruit flies can be used as a model for the visualization of Homologous chromosome-templated repair. Mutants with white eyes were first seen. The flies displayed large red patches across their eyes, a sign that the cell's DNA repair machinery had succeeded in reversing the mutations using the functional DNA from the other chromosomes.
The new system was tested with a single strand of DNA instead of the two that were in it. The authors found that the nicks gave rise to a restoration of red eye color similar to that of healthy flies. They found a 50%- 70% repair success rate with the nickase compared with just 20-30% in dual-strand cutting. Roy, the lead author of the study, said that he couldn't believe how well the nickidase worked. The system's flexibility could serve as a model for fixing genetic defects in mammals.
Guichard doesn't know how this process will translate to human cells or if we can apply it to any genes. It may be necessary to adjust to get efficient HTR for disease-causing genes.
The new research extends the group's previous achievements in precision-editing with allelic-drives, which expand on principles of gene-drives with a guide RNA that directs the CRISPR system to cut undesired variant of a genes and replace them with a preferred version of the genes.
A key feature of the team's research is that their nickase-based system causes less on- and off-target changes. One-time deliveries may be more beneficial than a slow, continuous delivery.
"Another advantage of this approach is that it's very easy to understand," said Bier. It relies on very few components and the DNA nicks are soft.
Roy said that if the frequencies of such events could be increased, such strategies could be used to correct many disease-causing genes.
More information: Sitara Roy et al, Cas9/Nickase-induced allelic conversion by homologous chromosome-templated repair in Drosophila somatic cells, Science Advances (2022). DOI: 10.1126/sciadv.abo0721. www.science.org/doi/10.1126/sciadv.abo0721 Journal information: Science Advances