Even though the appetite for data continues to rise, the cost of a full genome is still less than $1,000. The economy may be further accelerated by the claims of reducing that cost by another order of magnitude to $100.
The UG 100 can perform a complete human genome in about 20 hours, with precision comparable to existing options, but does so at a far lower cost.
I won't attempt a full explanation of the technical advances because they aren't entirely understandable to people who aren't experts. It's important to understand that the DNA is amplified in a reagent and then passed through small channels where it can be imaged by a lot of base detectors. The whole genome is reassembled by matching the ends of the sequence.
The claimed advance is threefold. Instead of having the reagent travel down fluidic channels that must be flushed afterwards, the micromachinery is etched onto a 200mm Silicon wafer. This process uses cheap stock and can be mass manufactured.
It allows the reagent to be deposited in the center of the wafer, which spins to distribute it evenly across its entire surface. The resulting sequence can be read during rotation of the wafer, similar to reading a compact disc.
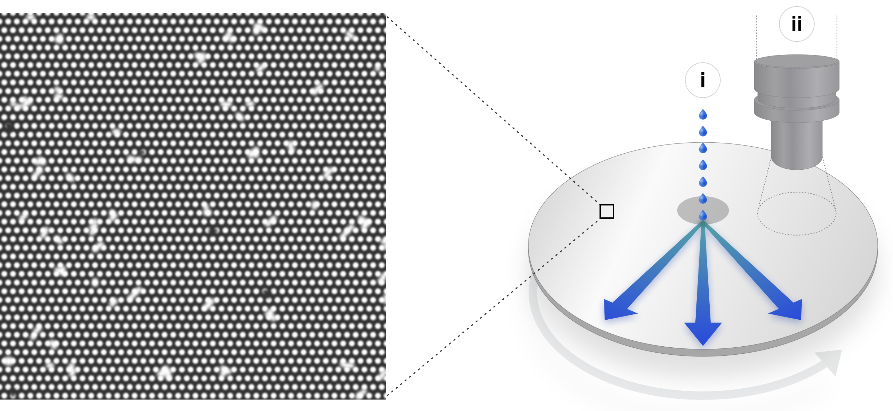
An image of the wafer's micropatterned surface is shown in the diagram of the open water process.
The second advance is a little more arcane, having to do with the process of preparing and directly reading the DNA, rather than replacing the bases with more machine-readable ones or relying on dicey particle-level imagery. It's less destructive to the original strands and doesn't require error-prone measurements like individual photon counts.
Machine learning is used to speed up the process of turning optical data into usable data. A deep neural network trained on multiple genomes and fragments is set to verify and assemble all the tiny pieces of data into the whole genome. This process speeds things up.
The size and density of the wafer and its surface are some of the factors that lead to improved throughput. For now, a 90% reduction is enough to push the price lower.
The company is currently working with early access partners to put out some early proof of concept studies showing the capabilities of the sequencing technique, according to the founder and CEO. The first of these collaborations with the Broad Institute and others are being presented soon or currently available as preprints.
Broader commercial deployment is expected in 2023, with final pricing likely to reflect the advantage conferred by this method over others. I asked Almogy what the areas of the medical industry that will benefit the most from this new capability were.
He said that he believes that genomics will be the first line of diagnostic across diseases.
We are talking with partners who are interested in doing more genomes, but also RNA expression and proteomics. This is important in epigenetic studies that look at how our genes change as we age.
Isabl is way ahead of him on the use of genetic profiling to fight cancer, and it may be one of the earliest clinical applications. The company could make it even quicker.
Isabl’s rapid whole-genome analysis opens the playbook for cancer treatment
The cost of single cell sequencing prevents us from routinely using it for applications like immune profiling. Reducing the cost could change the equation.
If you wanted to have it covered by insurance, you could get it done monthly if you wanted to, but the industry seems to be on the verge of another data explosion, beyond the scale of the one we are already in. Management and utilization of the newly deepened sea of information is likely to be the next opportunity with companies like Ultima increasing their data volumes.