Novel technologies will turn sunlight into electricity and fuels. These materials are a reality because of the combination of molecule and nanoparticles. The materials absorb sunlight and give electrons to the nanoparticles. The fuel is produced by the reactions that occur when the electrons are 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- 888-609- Researchers hope that this process will work. Scientists have found a way to track the electrons as they travel from molecule to molecule. If, where, when, and why electrons get stuck can be measured by researchers. It is important to find better combinations for innovative materials.
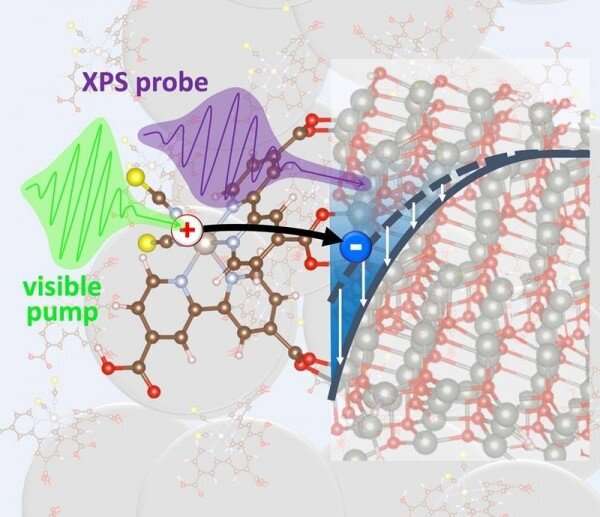
A new tool can be used to follow electrons traveling between molecule and nanoparticles that convert sunlight into electricity or fuels, according to a study published in The Journal of Physical Chemistry Letters. It turns out that zinc oxide is a very common material that stalls the electrons for a while. The electrons can only move on the surface of the nanoparticles. It is likely that the charges can be lost or damaged. The charges should travel straight through the nanoparticles. Researchers will be able to design better materials for turning sunlight into other forms of energy thanks to the ability to reveal the bottlenecks for electron travel.
To turn sunlight into electricity or fuel, a material must absorb the light and direct the light energy to electrons. The electrons must move around to form a current. One way to achieve both steps is to use molecules that are very good at catching sunlight and attaching them to something that is very good at moving electrons around. The electrons can move around inside zinc oxide more easily than in other materials. Despite this fact, zinc oxide electrodes wouldn't work as well as other zinc oxide ones. What is happening?
The Advanced Light Source is a Department of Energy (DOE) Office of Science user facility that uses a technique called time-resolved X-ray photoelectron spectroscopy. The electrons are stuck between the zinc oxide and the molecule. The material pushes the electrons toward the surface. If the electrons were able to travel straight through the bulk, they would be trapped more easily. The study helps explain why zinc oxide is not as effective as it could be. A new testing scheme for future materials is provided by it.
More information: Stefan Neppl et al, Nanoscale Confinement of Photo-Injected Electrons at Hybrid Interfaces, The Journal of Physical Chemistry Letters (2021). DOI: 10.1021/acs.jpclett.1c02648 Journal information: Journal of Physical Chemistry Letters Citation: Decoding the lifecycle of photogenerated charges (2022, April 29) retrieved 29 April 2022 from https://phys.org/news/2022-04-decoding-lifecycle-photogenerated.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.