A research group used inexpensive elements to demonstrate the feasibility of making materials for LIBs. This method could reduce dependence on rare metals.
Their results were published in the journal Applied Energy Materials.
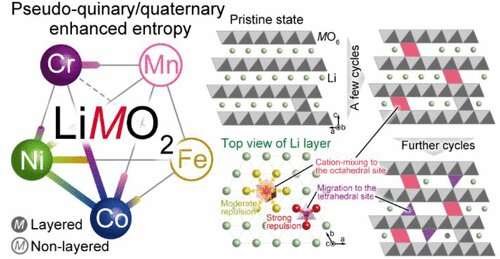
The key component of LIBs is the crystal structure of rare metals. In these materials, it's easy to extract and insert lithium.
Inexpensive elements have long been sought after by scientists. Only a small amount of salt can be dissolved into water.
A different strategy was employed by the research group led by professor Ichitsubo. They harnessed the energy gain from the configurational entropy and expanded the elements. The use of nickel and cobalt was reduced.
Ichitsubo says that the approach unlocks the potential of other unused elements and will enable us to maximize multiple electrode properties simultaneously.
The new method may improve the safety of LIBs. The author of the paper states that increasing configurational entropy raises the stabilization of the electrode material and contributes to the safety of the whole battery.
Ichitsubo and his group explained how the battery cycle is affected by these new materials. The high-entropy strategy will serve as a guideline for developing novel high- performance materials.
The ability to synthesise new materials opens up new avenues of research into LIBs, as the cyclability and capacity did not match conventional LIBs.
More information: Tomoya Kawaguchi et al, Influences of Enhanced Entropy in Layered Rocksalt Oxide Cathodes for Lithium-Ion Batteries, ACS Applied Energy Materials (2022). DOI: 10.1021/acsaem.1c03968 Citation: Researchers unlock potential means to reduce reliance on rare metals (2022, April 28) retrieved 28 April 2022 from https://phys.org/news/2022-04-potential-reliance-rare-metals.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.