A discovery made by Michigan State University researchers could have big implications for biology.
The team reported in the journal eLife on a new way that nature can switch off important proteases.
Evolutionary biologists say that this type ofProtein is highly conserved, which is how the researchers made this finding.
These types of proteases are found in organisms that span the kingdoms of life. The best-known member of this family is implicated in Alzheimer's disease, and it was discovered in humans in 1997.
The first example of regulating an intermembrane protease with a natural inhibitor is shown in the paper.
Will it tell us how to change it? It could give people ideas on decorations they could put on to try as therapies.
It will take years for this information to be used to design drugs to treat Alzheimer's. The bacterium behind the infections of anthrax and other diseases are included.
According to the American Association for the advancement of Science, which has a fellowship with him, many of thebacteria have the same proteases that are related to the one we studied.
eLife noted that a better understanding of these proteases could help develop applications in other areas. It helps paint a more complete picture of how life works.
Petra Anne Levin, an editor for eLife and a professor of biology at Washington University in St. Louis, wrote that the work should have a broad impact.
There are scissors, spores, and Corvettes.
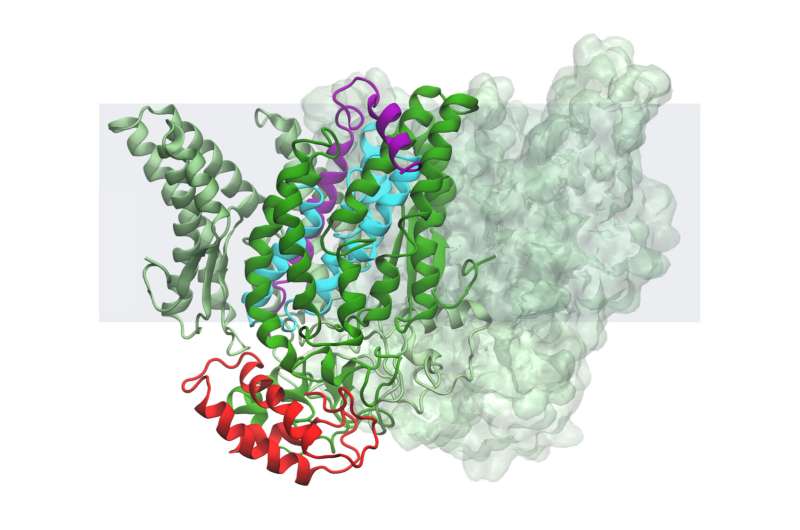
A protease is a type of machine that nature uses to chop up other proteins. Cells use this process to achieve a variety of goals. The active site of an intermembrane protease is buried inside a cell.
Sometimes you will hear them referred to as "scissors in the membrane."
The researchers studied the protease and found that it is part of the biological system that B. subtilis uses to make spores. One of the reasons these pathogens are so persistent is that they can weather harsh conditions and then reactivate once things improve.
It has been difficult for researchers to determine exactly how the proteases work. Adding to the complexity of the project, the researchers thought their protease might be working in a way that had never been documented before.
The system has evolved a lot when you look at other related organisms. It has high-end machinery.
Extensive genetic and biochemical testing was led by a PhD student in the lab. Olenic earned her PhD after completing this project.
The results of the experiments Olenic and Kroos ran wouldn't provide all the answers they were looking for. They turned to a long-time colleague, Michael Feig, an MSU professor of biochemistry and molecular biology, to bring in computer modeling and help complete the expansive puzzle.
A research associate in the lab had expertise in a technique that could predict structures. The technique has gained more attention recently due to the development of software packages that make it more accessible to the scientific community.
Heo and Feig had the know-how to help Kroos and Olenic figure out how the protease worked.
This is a really cool story and a nice collaboration that took a lot of hard work and perseverance. It is a credit to the two of them that they did a nice piece of work with the modeling.
The puzzle has been completed.
The team's results show that the B. subtilis is kept inactive with help from two other proteins. One of the inhibitors works like a clamp, keeping the other in the active site.
The researchers theorize that the bacteria can cause the protease to work by releasing the clamps and allowing the blocking protein to slip out.
This was like putting together a 5,000-piece jigsaw puzzle without knowing what it looks like. The team has enough data and results to be confident that it has a reasonable model of how things work. The researchers are not done there.
Their next step is testing the predictions of the model and seeing how well they match reality. Two undergrad researchers are in the lab.
X-ray crystallography and cryogenic electron microscopy are used to determine the structures of the proteins. The part has been worked on for about a decade with the help of the team at MSU.
He thinks he may retire before the puzzle is complete. If his colleagues will let him, he might try to sneak back and help. He is excited to see how much of the puzzle he and his team can solve with a few years left.
Science didn't know about the proteases when Kroos started. After their 1997 discovery, Kroos decided he needed to switch his research focus to include these proteins.
Not everyone believed that the switch could be made, so it was not an easy thing to do. He was supported and collaborated with at MSU.
He said that the department is strong in the field of membrane proteins.
That hypothesis was confirmed.
More information: Sandra Olenic et al, Inhibitory proteins block substrate access by occupying the active site cleft of Bacillus subtilis intramembrane protease SpoIVFB, eLife (2022). DOI: 10.7554/eLife.74275 Journal information: eLife Citation: Recent research on intramembrane proteases could lead to new Alzheimer's treatments (2022, April 26) retrieved 26 April 2022 from https://phys.org/news/2022-04-intramembrane-proteases-alzheimer-treatments.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.