MIT chemists have created a new chemical reaction that allows them to synthesise a ring of alkenes using a catalyst.
Their reaction, which yields a ring containing two carbon atoms and one phosphorus atom, can be performed at normal temperature and pressure, and uses a novel molecule that supplies the phosphorus atom.
Christopher Cummins, the Henry Dreyfus Professor of Chemistry at MIT and the senior author of the paper, says that this is a rare example of a discovery of a new catalytic reaction and that it opens up a wealth of new opportunities to do chemistry.
Cummins says these rings could be used as catalysts for other reactions or as a way to make useful compounds.
The paper was published in the Journal of the American Chemical Society. The author of the study is a former MIT research fellow.
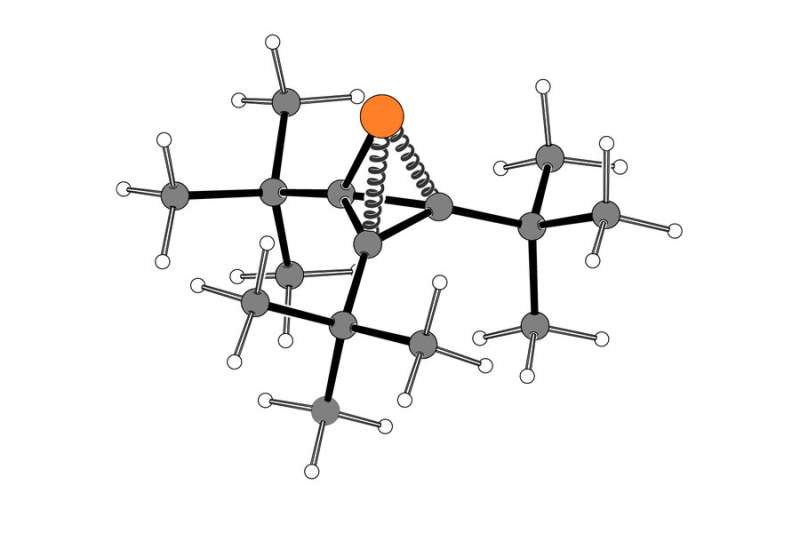
A ring is being created.
Many industrially useful chemical reactions can be made with organic compounds that contain double bonds between carbon atoms. Researchers can create a wide variety of new products by breaking carbon-carbon bonds and adding new atoms.
Chemists have previously created ways to convert a carbon-carbon double bond into a three-membered ring by adding either another carbon atom, a nitrogen atom or an oxygen atom. The compounds can be found in many products.
It has been difficult to find a way to incorporate it into olefins without using brute force because it is heavier than carbon, nitrogen, or oxygen. The MIT team wanted to use a catalyst to transfer a phosphinidene group to an organic chemical group.
They needed a starting material that could act as a source of phosphinidene, but they didn't have any that were stable with phosphorus.
In a paper published in the year 2019, Cummins's lab was able to come up with one possible source, consisting of phosphinidene attached to a molecule that contains several hydrocarbon rings. They were able to synthesise a three-membered ring with this compound, but it took high temperatures to work with certain types of olefins.
In their new paper, the MIT team used a different source of phosphorus for the reaction than the Cummins lab had first synthesized. The shape of this molecule is like a compressed spring because of the small bond angles between the four atoms.
The compound, called tri-tert-butylphosphatetrahedrane, has three vertices with carbon atoms attached to a chemical group called tert-butyl and one with an unshared pair of electons. The strained molecule can be broken apart under certain conditions.
Synthesis is efficient.
The researchers were able to create three-membered rings using a nickel-based catalyst. This reaction can be done at room temperature.
Cummins says that all the stars aligned in terms of us being able to synthesise a highly strained precursor that leads to room temperature reactivity and rapid catalysis.
The researchers believe that this reaction is dependent on phosphinidene being temporarily transferred to the nickel catalyst complex. The double bond of the olefin is then incorporated into the catalyst.
They hope to explore the possibility of creating a variety of new compounds that include the ring, and to develop ways to control which of two mirror image versions are synthesized. Adding more molecule to the rings will open them up to create other useful compounds. Potential applications for these kinds of products include catalysts for other reactions.
More information: Martin-Louis Y. Riu et al, Reactions of Tri-tert-Butylphosphatetrahedrane as a Spring-Loaded Phosphinidene Synthon Featuring Nickel-Catalyzed Transfer to Unactivated Alkenes, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c02236 Journal information: Journal of the American Chemical SocietyThe story was re-posted by MIT News, a popular site that covers news about MIT research, innovation and teaching.
Citation: 'Spring-loaded' system pops phosphorus into molecular rings (2022, April 21) retrieved 21 April 2022 from https://phys.org/news/2022-04-spring-loaded-phosphorus-molecular.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.