When she joined the lab of Prof. Fleishman, she wanted to break down plastic waste into useful chemicals. Dead trees can be recycled by white-rot fungi, which degrades wood into a substance that returns to the soil. Why not get the same enzymes to degrade the waste?
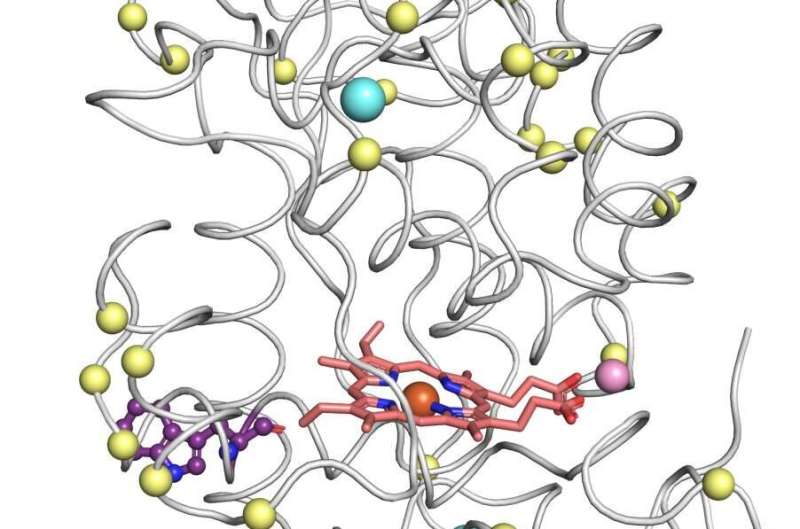
Fleishman says that the natural peroxidases are difficult to work with. Over the past few years, his lab has developed computational methods that are being used by thousands of research teams around the world to design enzymes and other proteins with enhanced stability and additional desired properties. For such methods to be applied, the precise structure of aprotein must be known. This typically means that the crystals must be sufficiently stable to form, which can be bombarded with X-rays to reveal their structure in 3D. This structure is then altered using the lab's methods to create an improved version of the original. Attempts to improve peroxidases can run into a dead end if the original is too fragile to form crystals, as is the case with versatile peroxidases.
Her timing was perfect, as she took a chance on the prima donna enzymes. Since the 1980s, attempts have been made to get around the need for crystallization by predicting a 3D structure from the DNA sequence, but these predictions were unreliable for complex proteins. After several weeks after embarking on her project, Barber-Zucker's predicted enzyme structures suddenly looked reliable. At that time, DeepMind and several university research teams had improved their artificial intelligence-based structure prediction methods to the point where they were highly accurate. The approach has led to predicted models that are nearly as accurate as those obtained with crystallography.
With the new structures, Barber-Zucker and colleagues were joined by students in Fleishman's lab. A team of experts took about a decade to describe one of the peroxidase family's genes. Within less than six months, Barber-Zucker and colleagues were able to design, produce and analyze stable versions of three versatile peroxidases, which had not previously been produced in the lab. The scientists used 3D models to start. The models were applied to by Fleishman's lab, called theProtein Repair One Stop Shop, or PROSS, which is a software program that modifies a computer's function on demand.
Fleishman says that this combined approach opens an enormous range of opportunities. He is referring to the fact that less than 0.05 percent of the millions of natural proteins whose DNA sequence is known have been solved for 3D structures in the lab. The first question we used to ask was, "Do we have a structure of theProtein we want to focus on?", but now this question has become irrelevant, we can manage with a structure or without, and that is a true turning point.
Drug design could benefit from this advance. A laborious process that involves crystallization and altering numerous regions of the animal molecule is needed to adapt lab animals to humans. This and other antibody engineering processes are expected to be much more efficient thanks to the new advance.
The original rationale for this study is promising. For example, wood-degrading enzymes could be adapted to recycle agricultural waste. It may be possible to break down waste into sugars that can be used to make fuel, instead of burning it or dissolving it with chemicals. Farmers would be able to recycle in small bioreactors.
The enzymes could be used to degrade pollutants. Barber-Zucker has shown that her improved enzymes can attack a dye. She found that each of the three improved enzymes had different activity in the lab, and that each specialized in degrading different wood components, which suggests that they may act together. The stability and resistance to heat of the three enzymes is an essential feature for their use in industry. Barber-Zucker wants to develop an enzyme cocktail in which a dozen different enzymes, including her versatile peroxidases, will work together to break down waste wood into useful materials.
She says that her vision of recycling hard plastics using these enzymes may become a reality in the near future.
The Journal of the American Chemical Society published the research.
More information: Shiran Barber-Zucker et al, Stable and Functionally Diverse Versatile Peroxidases Designed Directly from Sequences, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.1c12433 Journal information: Journal of the American Chemical Society Citation: AI gives algorithms the means to design biomolecules with a huge range of valuable functions (2022, April 13) retrieved 13 April 2022 from https://phys.org/news/2022-04-ai-algorithms-biomolecules-huge-range.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.