Two thousand ventilators in UK hospitals are at risk of suddenly shutting down due to electrical faults that have led to a global safety alert.
Hospitals have been ordered to source replacement ventilators after a company said that some of its breathing support devices could suddenly stop working.
A number of electrical faults in the devices can result in an unexpected shutdown, which can lead to loss of ventilation, according to the Medicines and healthcare products Regulatory Agency.
There have been five reported cases of shutdowns in the UK so far, none of which involved patient harm. There have been 389 reports of failures, including one where a patient died and four where they were seriously injured. The warning alarm didn't sound in six of the cases.
Several manufacturers increased production of ventilators during the Pandemic. In response to Covid-19, the MHRA brought in a fast approval process for ventilators and other medical devices.
The affected machines are used to support patients in critical care and high-dependency units.
The root cause of the problem was not yet known and there was no permanent solution for it, according to the MHRA.
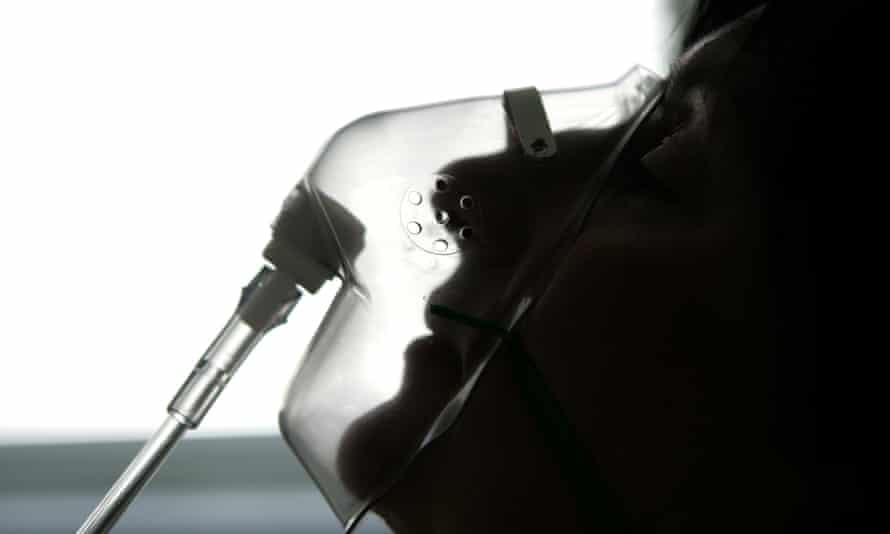
The Dutch medical equipment company said it was not advising customers to remove affected devices because of the low incidence of problems, and instead recommended steps to lower the risks, such as connecting ventilators to a remote alarm system.
Hospitals and healthcare providers have been ordered by the MHRA to source alternative ventilators and train staff to use them, and affected devices should be removed from use by the end of May. Doctors need to increase monitoring of patients and make sure a backup is always available.
If there was a risk of severe patient harm due to the lack of availability, hospitals would still be able to use the affected ventilators.
Hypoxic conditions can result in long-term cognitive impairment to the patient, if undetected by healthcare professionals. There is a risk of death if a patient is not breathing for a long period of time.
There are a number of problems with the Philips Respironics ventilators. An issue that could lead to device shutdown was reported in the US in January. There was a production issue that involved an expired glue and only affected a particular batches.
In September 2020, the MHRA issued a recall of about 300 Philips ventilators in the UK because they were at risk of suddenly stopping working. The company said that the issue was related to V60 ventilators with a certain printed circuit board assembly.
In June 2021, a separate alert was issued due to the degradation of foam used in some ventilators and sleep apnoea machines, which could lead to users inhaling cancer-causing chemicals. About 105,000 devices worldwide and 2,000 in the UK are affected by the latest safety alert for the V60, V60 Plus and V680 Philips ventilators. The Department of Health is able to provide replacement ventilators to hospitals.
The devices were used in critical care units and the recall was significant.
He said it was frustrating because they are outstanding machines. He said that if the cause of the problem could not be identified, they would not fix it.
Helen Hughes, chief executive of the Patient Safety Learning charity, said there was a significant patient safety concern about some of the devices.
As a result of the safety notice, the company apologized to any patient or healthcare provider who may have experienced any concern. The team has been set up to deal with the reported shutdowns.
The V60 ventilator has been in use for almost 10 years with a high reliability record. If there is a reported issue, we will take each individual complaint seriously, address it and report it to the competent authorities.