Depending on where you live, and the persistence of public health rules like masking and vaccination entry requirements, it feels like the beginning of the end of COVID-19, or maybe the end of the end. While some have made the decision to “move on” after more than two years, there’s still a lot of COVID-19 being spread around, along with the continued problem of getting populations vaccinated. Recognizing that disease control will continued to be challenged by new variants, waning immunity, possible vaccine escape, and continued vaccine hesitancy (where vaccines are widely available), we continue to need good measures for preventing or controlling disease.
One of the earliest randomized controlled trials that studied the effects of vitamins on health was published a year into the Pandemic. In addition to its effects on muscle and bone, vitamins D and D3 are thought to support immune function. It's common for older adults to be deficient in vitamin D because they live in areas where there isn't enough sunlight. There has been a lot of interest in the possible role of vitamins D and D2 in helping to raise the levels of vitamins D and D2 in the body. This was the case before the arrival of COVID-19, and interest grew in the early days of the Pandemic.
There have been trials that have either been negative or positive. There is no clear, consistent benefit. The challenge with observational data is that it's difficult to tell if a deficiency of the D is causing more illness or if it's just a sign of poor overall health status. This shows the importance of prospective trials with randomization. A year ago, I wrote about a study that showed a single dose of 200,000 IU would be equivalent to taking 200 tablets of the standard 1000 IU tablet strength. The placebo had no effect on any of the outcomes that were measured.
The new trial, which has been published as a preprint, was recently discussed on the internet by an epidemiologist. David A. Jolliffe is from a number of centers in the United Kingdom. There is a Phase 3 randomized controlled trial for the prevention of Covid-19 or other Acute Respiratory Infections. The COVIDENCE cohort study was established in May 2020 with the goal of identifying risk factors related to COVID-19 infections.
This was a three-arm study. To be included in the trial, you had to be 16 years of age. If you had a small number of medical conditions or were already taking vitamins, you were excluded. 6,200 patients were assessed and randomized.
Participants were asked to complete a monthly follow-up survey about respiratory infections, exacerbations of asthma or chronic obstructive pulmonary disease, COVID-19 symptoms, and adverse effects. The six-month follow-up was sent in June 2021. A group of people who were randomized but did not receive treatment were offered a chance to have their vitamin D tested.
The proportion of participants that developed at least one acute respiratory illness was the primary endpoint. Secondary endpoints are included.
The incidence of death, serious adverse effects, and high levels of calcium were measured.
Table 1 has a snapshot of baseline characteristics. The median age of participants was 60 with a fairly wide age range, mostly female, and overwhelmingly White.
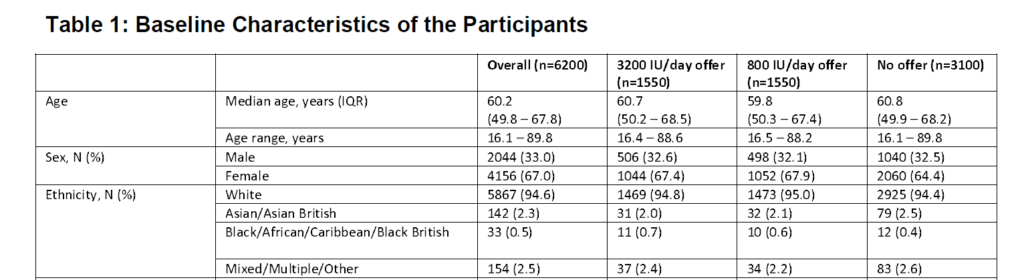
Only a small percentage of participants had beenvaccinated at the time of recruitment.
The mean 25(OH)D was 39.7nmol/L, and 97.4% were below the threshold. Almost all of the people randomized to the treatment groups consented to the test.
The six months were from December 2020 to June 2021. Almost all of the people received at least one dose of the vaccine. 99.9% of participants reported taking their vitamins at least 6 days per week. There were significant increases in the levels of vitamins D and E in those who took the supplements.
In a sensitivity analysis where non-adherent participants were excluded, the differences were greater.
Did vitamins D prevent respiratory infections? No. There were no significant differences in the proportion of people who experienced at least one confirmed acute respiratory infection. The groups of vitamins D look worse with some of the measures. There were no differences in the incidence or severity of COVID-19 infections, the duration of related symptoms, or any other measure.
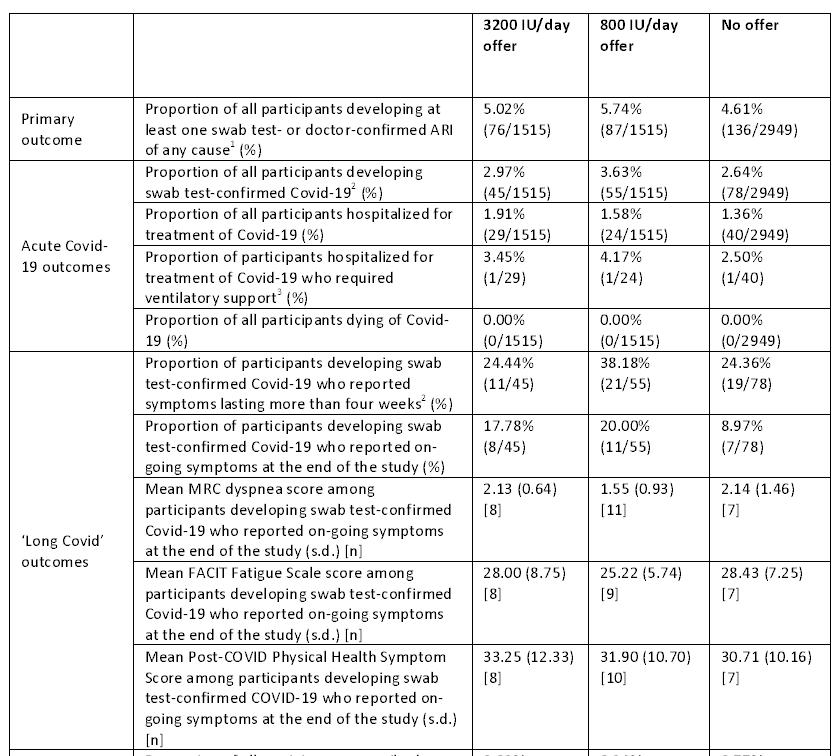
There was no evidence that vaccination changed the effects of the allocation. The primary, intention-to-treat, analysis did not reveal any differences compared to the sensitivity analysis. The results didn't change even though the patients in the control group took vitamins. The results didn't change even if the patients in the treatment groups were not compliant with therapy.
There were no adverse events. Some patients in the high-dose group developed hypercalcemia and had their symptoms resolved with the discontinuation of vitamins D and E.
The study had several strengths, including the baseline low levels of vitamins D and E and the effectiveness of the supplements. This was a more typical daily dose compared to a single massive dose that I wrote about a year ago. There were a few dropouts and a small number of COVID-19 cases. We need to be careful in making conclusions because of the limited number of cases. There was no suggestion of benefit.
There was no effect on the incidence or consequences of COVID-19 or other acute respiratory infections in this randomized controlled trial. This was despite the fact that active treatment with vitamin D raised levels.
This is a trial that will show no effect on the prevention of acute illness. Having said that, it is clear that there is a widespread deficiency of vitamin D in this population. There is no convincing evidence to suggest that you can protect yourself from COVID-19 infections by taking vitamins D and D3.
Scott Gavura is committed to improving the way medications are used and examining the profession of pharmacy through the lens of science-based medicine. He wants to improve the cost-effective use of drugs at the population level. Scott has a Bachelor of Science in Pharmacy degree and a Master of Business Administration degree from the University of Toronto. His professional background includes pharmacy work. He is a registered pharmacist in Canada. There are no conflicts of interest for Scott. All views expressed by Scott are his own, and do not represent the opinions of any current or former employers, or any organizations that he may be affiliated with. All information should not be used as a substitute for consultation with a licensed and accredited health professional.
All posts can be viewed.

You can buy an e-book.
Dr. Hall has a video course.
The text is powered by the internet.
Powered by TranslatePowered by
TranslatePowered by  Translate
Translate