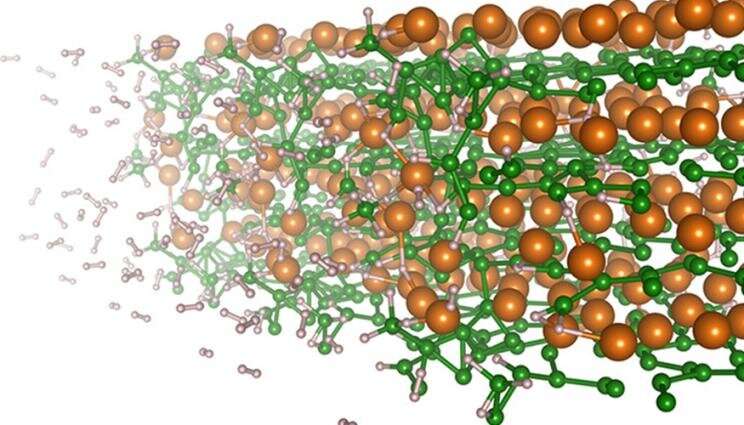
Scientists at Lawrence Livermore National Laboratory have discovered why hydrogen slows as the material absorbs hydrogen, providing insight that could be used for improvements.
Improving hydrogen storage in solid-state materials depends on a better understanding of multistep chemical reactions. The material becomes a hydrogen-saturated phase when it reacts with hydrogen and rearranges itself. There are a variety of chemical and electrochemical energy-storage contexts.
The underlying mechanisms involved in the hydrogenation of magnesium diboride have been revealed by a team of scientists. They found that magnesium ion drive the electric polarization of the atoms and that charge redistribution is critical for the formation of the hydrogen-saturated Mg(BH 4 ) 2 phase. The positively charged center boron atom can attract and bind with hydrogen anions, which are negatively charged through interactions with Mg 2+. There is research in the journal.
The analysis showed that the formation of Mg(BH 4 ) 2 prevents full hydrogenation without high temperature and pressure in experiments. Boron is more prone to bind hydrogen when the environment is poor because it is less stable. The surface of the remaining MgB 2 material becomes more Mg-rich as the material transforms to Mg(BH 4 ) 2.
The simulations capture the reaction pathways that lead to hydrogen absorption.
Alexander Baker and Brandon Wood are also authors.
More information: Keith G. Ray et al, Understanding Hydrogenation Chemistry at MgB2 Reactive Edges from Ab Initio Molecular Dynamics, ACS Applied Materials & Interfaces (2022). DOI: 10.1021/acsami.1c23524 Journal information: ACS Applied Materials and Interfaces Citation: Hydrogen storage reactions bear a complex dance toward faster uptake (2022, March 30) retrieved 30 March 2022 from https://phys.org/news/2022-03-hydrogen-storage-reactions-complex-faster.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.