Researchers at Cornell University have found that a nitrogen-doped, carbon-coated nickel anode can be used in hydrogen fuel cells at a fraction of the cost of precious metals.
Hydrogen fuel cells hold great promise as efficient, clean energy sources for vehicles and other applications, and the new discovery could accelerate the widespread use of hydrogen fuel cells.
It is one of a number of discoveries made by the lab in their search for catalysts for use in alkaline fuel cells.
This finding makes progress toward using efficient, clean hydrogen fuel cells in place of fossil fuels, according to Abru, the Emile M. Chamot Professor in the Department of Chemistry and Chemical Biology.
The results of A Completely Precious-Metal-Free Alkaline Fuel Cell With Enhanced Performance Using a Carbon-Coated Nickel Anode were published in the Proceedings of the National Academy of Sciences.
Platinum and other expensive precious metals are required in hydrogen fuel cells to make electricity. APEMFCs lack the necessary performance and durability to replace precious metal-based systems.
A fuel cell can produce electricity through two reactions. Platinum is a model catalyst for both reactions because it is efficient and durable in the acidic environment of a PEM fuel cell.
What about other materials?
The researchers wrote that recent experiments with non-precious-metal HOR electrocatalysts aimed to overcome two major challenges: low intrinsic activity from too strong a hydrogen binding energy and poor durability due to rapid passivation from metal oxide formation.
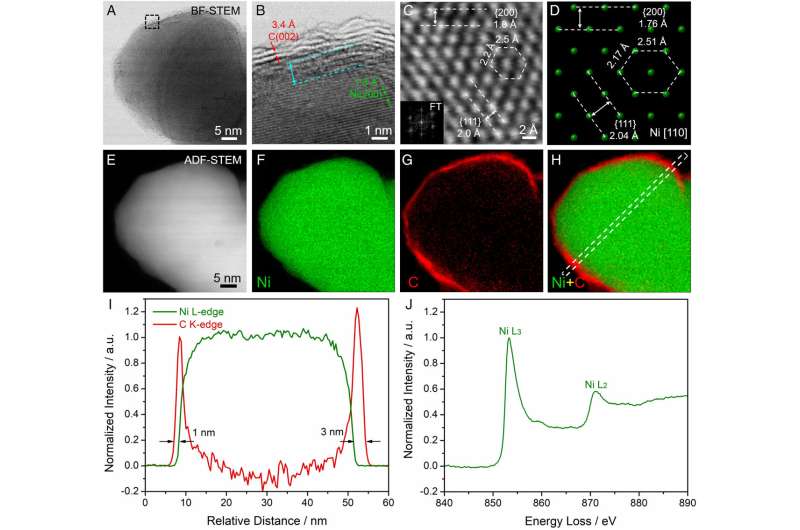
To overcome these challenges, the researchers designed a nickel-based electrocatalyst with a shell made of nitrogen-doped carbon.
The hydrogen fuel cell has a catalyst that is surrounded by a carbon shell. The precious metal-free hydrogen fuel cell outputs more than 200 kilowatts per square centimeter when combined with a cobalt-manganese cathode.
The hydrogen oxidation reaction is slowed by the presence of nickel oxide species on the nickel electrode. The nitrogen-doped carbon coating makes the reaction quicker and more efficient.
The formation of nickel oxides is prevented by the presence of the Graphene coating on the nickel electrode. The carbon monoxide is much more tolerant than the Platinum.
The use of this novel anode would dramatically lower prices, allowing the application of alkaline fuel cells in a wide variety of areas.
The co-authors are Francis DiSalvo, the John A. Newman professor of chemistry, and David Muller, the Samuel B. Eckert Professor of Engineering in the College of Engineering.
In February, Abru and colleagues, including DiSalvo, found that a catalyst made from a metal is nearly as efficient as a catalyst made from a metal.
More information: Yunfei Gao et al, A completely precious metal–free alkaline fuel cell with enhanced performance using a carbon-coated nickel anode, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2119883119 Journal information: Proceedings of the National Academy of Sciences Citation: Carbon-coated nickel enables a hydrogen fuel cell free of precious metals (2022, March 23) retrieved 23 March 2022 from https://phys.org/news/2022-03-carbon-coated-nickel-enables-hydrogen-fuel.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.