Ice cream can be enjoyable, except when it gets very hard because ice crystals have grown in it. Scientists say that a form of cellulose obtained from plants can be added to a treat to stop ice growth in the face of temperature fluctuations. The findings could be applied to the preservation of other frozen foods.
The results will be presented at the spring meeting of the American Chemical Society.
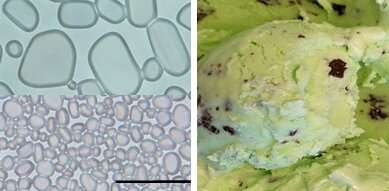
The ice cream has tiny ice crystals. During storage and transport, the ice can grow again. The recrystallization process causes smaller crystals to melt and the water to diffuse to join larger ones. The dessert takes on a grainy, icy texture that reduces consumer appeal if the ice crystals are bigger than 50 micrometers.
Some fish, insects and plants can survive in sub-zero temperatures because they produce antifreeze proteins that fight the growth of ice crystals. Adding ice cream to it is not practical because of the high cost of antifreeze and limited supply. Polysaccharides such as guar gum or locust bean gum are used instead. They sometimes work in one product but not in another. They want to clarify how they work and develop better alternatives.
He drew inspiration from them, even though he didn't use them in the study. They are amphiphilic, meaning they have a surface that repels water as well as a surface that has an affinity for water. He thought it was worth checking to see if they could stop ice crystal growth in ice cream. They are inexpensive, abundant and renewable because they are derived from the plant cell walls.
Min Li, a graduate student in the lab, says that the CNCs initially had no effect on the ice cream. Even though they were small, ice crystals were the same size. The researchers found that after the model ice cream was stored for a few hours, the ice crystals were completely shut down by the CNCs.
The tests showed that the ice recrystallization is possible with the help of the cellulose. The belief that stabilizers impede ice recrystallization by increasing the amount of water in the ice is incorrect.
When ice cream is stored in the supermarket and then taken home, the scientists found that the current stabilizers are not as protective as the CNCs. Slow-melting ice cream could be made using the Additive that slows the melting of ice crystals. The stabilizer would need to be reviewed by the FDA if it were to be shown to be safe at the levels needed in food.
It is possible to preserve cells, tissues and organs in biomedicine with further research.
More information: Inhibiting ice recrystallization by cellulose nanocrystals: Influences of sucrose concentration and storage time, ACS Spring 2022. acs.digitellinc.com/acs/live/22/page/677 Citation: Giving the cold shoulder to crunchy ice cream—with a dash of cellulose (2022, March 20) retrieved 20 March 2022 from https://phys.org/news/2022-03-cold-shoulder-crunchy-ice-creamwith.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.