Carbon is an amazing element. Carbon atoms are arranged in a way that makes them soft. Diamond is one of the hardest materials in the world.
Carbon is the key ingredient for most life on Earth, as well as the pigment that made the first tattoos, and the basis for technological marvels such as graphene, which is a material stronger than steel and more flexible than rubber. There is a Periodic Table of the Elements.
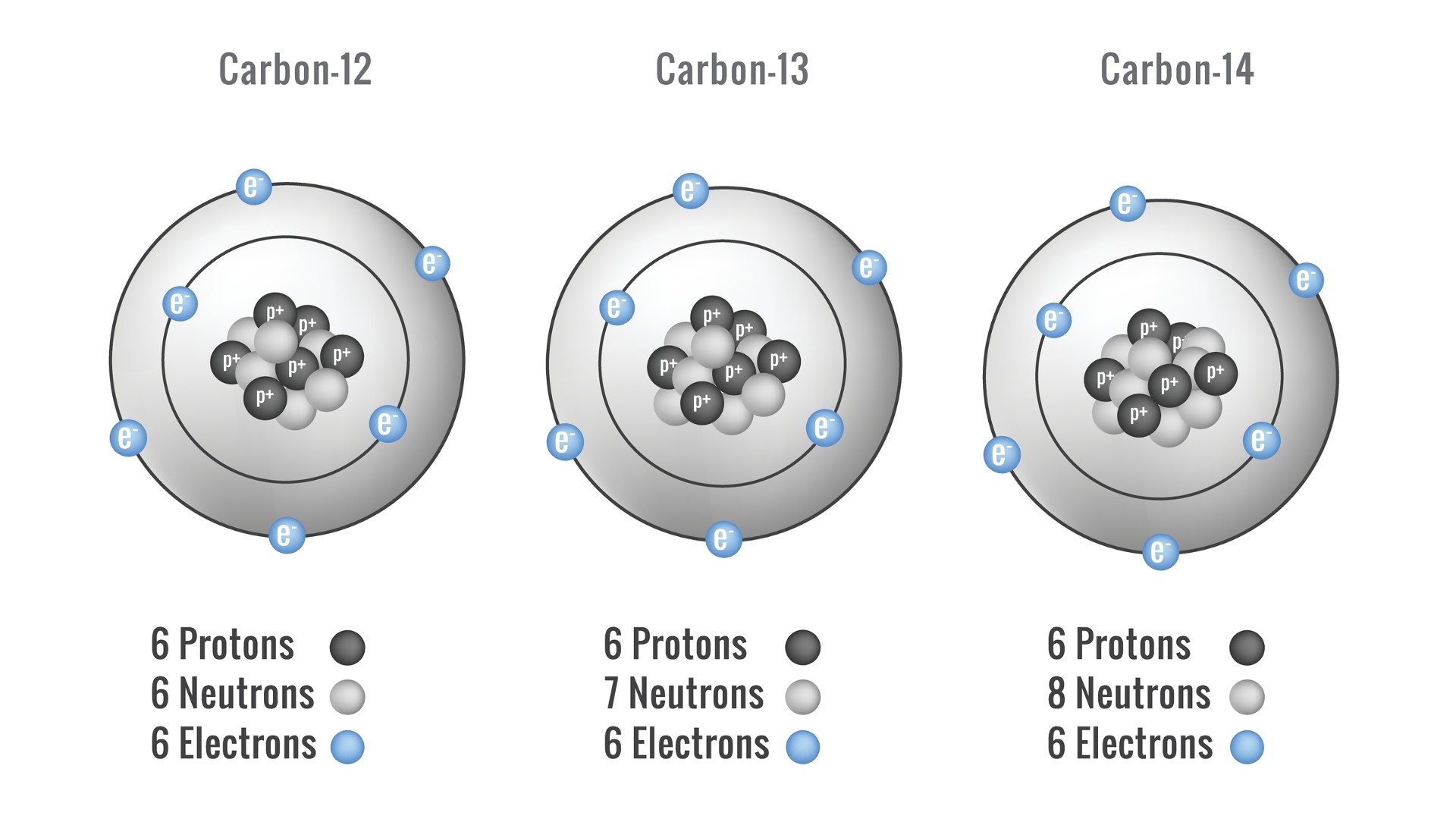
Carbon is made up of carbon-12, carbon-13, and carbon-14, which make up almost all of the carbon in the universe and are very important in dating organic objects.
The sixth-most abundant element in the universe is carbon, which forms in the belly of stars.
RECOMMENDED VIDEOS FOR YOU...
Older stars that have burned most of their hydrogen accumulate leftover helium. There are two protons and two neutrons in the nucleus. Under very hot temperatures greater than 100,000,000Kelvin (179,999,540.6 F) the helium nuclei begin to fuse, first as pairs into unstable 4-proton beryllium nuclei, and eventually as enough beryllium nuclei blink into existence. The end result was atoms with six protons and six neutrons.
A pattern is created by carbon. It can form long, resilient chains. It can bond with up to four other atoms. The nucleus of a atom is surrounded by a cloud of electrons. According to the University of California, Davis, chemists define the properties of atoms by what is in each shell. The first electron shell has two electrons and the second has four out of a possible eight spaces. electrons are shared in their shell when atoms bond. Four other atoms are able to bond to four other atoms because of the empty spaces in Carbon's outer shell. It can form double and triple bonds to fewer atoms.
Carbon has options. Nearly 10 million carbon compounds have been discovered, and scientists estimate that carbon is the keystone for 95 percent of known compounds. Carbon is crucial to almost all life because of its ability to bond with many other elements.
Carbon's discovery is lost in history. The element was found in the form of charcoal. According to the World Coal Association, carbon as coal is still a major source of fuel worldwide. Coal is a key component in steel production, as well as a common industrial lubricant.
Archaeologists use carbon-14 to date objects and remains. There is carbon-14 in the atmosphere. According to the Iowa State University Center for Nondestructive Evaluation, plants take it up in respiration, in which they convert sugars made during photosynthesis back into energy that they use to grow and maintain other processes. Animals incorporate carbon-14 into their bodies by eating plants. According to the University of Arizona, carbon-14 has a half-life of over 5000 years.
Scientists can use the half-life of carbon-14 to measure how long the organisms have been alive. This method works on organisms that were once alive.
Carbon is studied for a long time, but that doesn't mean there isn't more to discover. The element that our prehistoric ancestors burned as charcoal may be the key to next-generation tech materials.
A new form of carbon was discovered in 1985 by Rick Smalley and Robert Curl of Rice University. According to the American Chemical Society, the scientists created a molecule made of pure carbon by vaporizing graphite with lasers. The molecule was made of 60 carbon atoms. The buckminsterfullerene was named after an architect. The researchers who discovered the molecule won a prize in 1996. According to a study published in the Journal of Chemical Information and Modeling in 2009, buckyballs have been found to prevent the spread of HIV. The hardest non-crystalline material ever seen was found by researchers in China in 2021, when they compressed buckyballs.

Other new, pure carbon molecules, including elliptical-shapedbuckyeggs and carbon nanotubes, have been discovered. In 2010, researchers from Japan and the United States won a prize for figuring out how to link carbon atoms together using palladium atoms, a method that enables the manufacture of large, complex carbon molecules.
Scientists and engineers are using carbon nanomaterials to build materials. A 2010 paper in the journal Nano Letters reported the invention of flexible, conductive textiles dipped in a carbon nanotubes that could be used to store energy, paving the way for Wearable batteries, solar cells and other electronics. Chemical supply companies now sell the ink.
Perhaps one of the hottest areas in carbon research today is the use of Graphene. Graphene is a sheet of carbon. It is the strongest material and is still flexible. It conducts electricity better than copper. Scientists are still discovering new things. In 2020, researchers reported in the journal Nature Physics that they could make it magnetic by stacking graphene in the right way.
Researchers in April of last year reported that they could make large amounts of mass-produced Graphene using a kitchen blender. Scientists in the Netherlands created a mathematical model to guide large-scale production in 2020. Graphene could become a huge tech material if scientists can figure out how to make lots of it. Imagine gadgets that are paper-thin. Carbon has come a long way from diamonds.
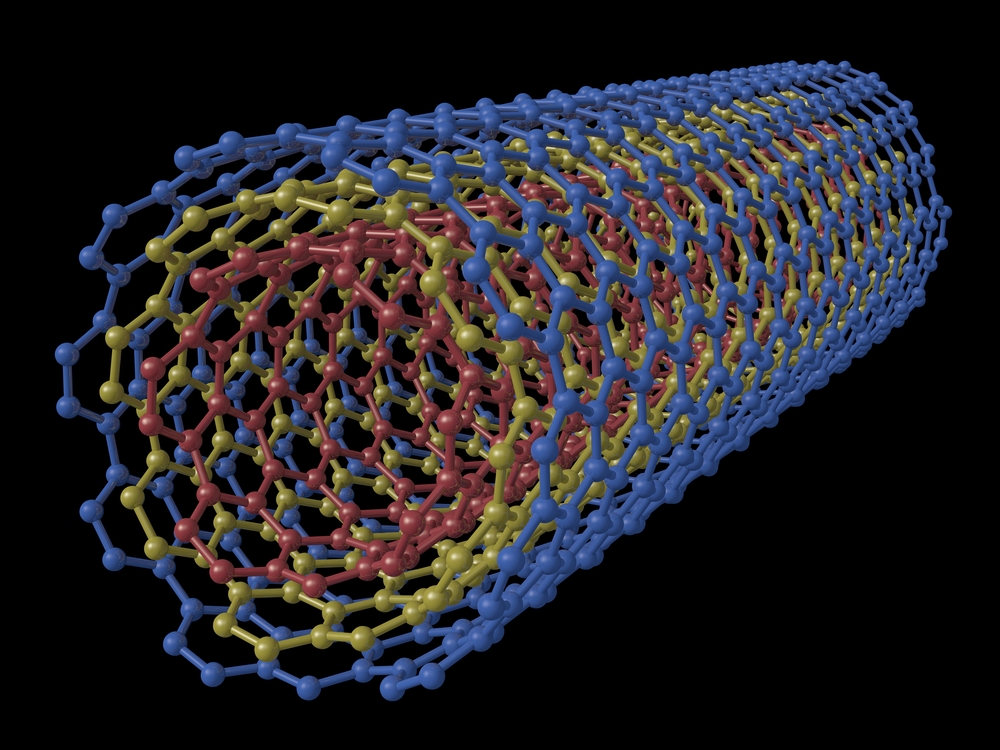
A carbon nanotubes is a structure made of carbon atoms. These tubes are used in a wide range of technologies. The tubes are so small that they are measured in nanometers. A nanometer is about 10,000 times smaller than a human hair.
Carbon nanotubes are 100 times stronger than steel, but only one-sixth as heavy, so they can add strength to almost any material.
The goal is to turn seawater into drinking water. Scientists at Lawrence Livermore National Laboratory have developed a process that can take the salt out of the water much more efficiently than traditional methods.
Under high pressure, traditional desalination processes pump in seawater. All large particles, including salts, are rejected by the membranes, allowing only clean water to pass through. The desalination plants are expensive and can only process a small amount of water.
In the study, the scientists mimicked the structure of a biological membranes, which is a matrix with pores. They used nanotubes that were 50,000 times thinner than a human hair. Only one water molecule can pass through the tube at a time, because of the small size of the nanotubes. The tube is too small to hold the salt ion.
The researchers think the new discovery has important implications for the next generation of water purification processes.
Additional reporting by a Live Science contributor.
H. King is on Geology.com. March 10, 2022.
Graphene research and their outputs: Status and prospect, was written by S.K., et al. The March 2020 edition of 5, No. 1 is here.
There are critical advances and challenges for the synthesis of carbon nanotubes and related materials.