A subfield of physics called quantum mechanics describes the behavior of particles.
During the first half of the 20th century, the results of quantum mechanics are often very strange.
Many of the equations of classical mechanics, which describe the movement and interactions of things at everyday sizes and speeds, cease to be useful at the scale of atoms and electrons.
In classical mechanics, objects exist in a specific place. In quantum mechanics, objects exist in a haze of probability, with a certain chance of being at point A, point B and so on.
According to the University of St. Andrew's in Scotland, quantum mechanics began as a set of controversial mathematical explanations for experiments that the mathematics of classical mechanics could not explain. It began at the turn of the 20th century, around the time Albert Einstein published his theory of relativity, a separate revolution in physics that describes the motion of things at high speeds. The origins of quantum mechanics can't be attributed to a single scientist. Multiple scientists contributed to a foundation that gradually gained acceptance and experimental verification between the late 1800s and 1930.
In 1900, German physicist Max Planck was trying to explain why objects at specific temperatures, like a light bulb, glowed in red. The equations used by Boltzmann to describe the behavior of gases could be used to explain the relationship between temperature and color. The problem was that Boltzmann relied on the fact that light and gas were made from tiny particles.
At the time, most physicists believed that light was a continuous wave and not a small packet. Einstein published a paper in 1905 that gave a boost to the idea of atoms and light.
Einstein suggested in his paper that the packet of energy could be absorbed or generated only as a whole when an atom jumps. Part of quantum mechanics comes from this location.
Einstein offered insights into the behavior of nine phenomena in his paper, including the specific colors that Planck described being emitted from a light bulb. The photoelectric effect is a phenomenon in which certain colors of light can cause electrons to be ejected from metal surfaces.
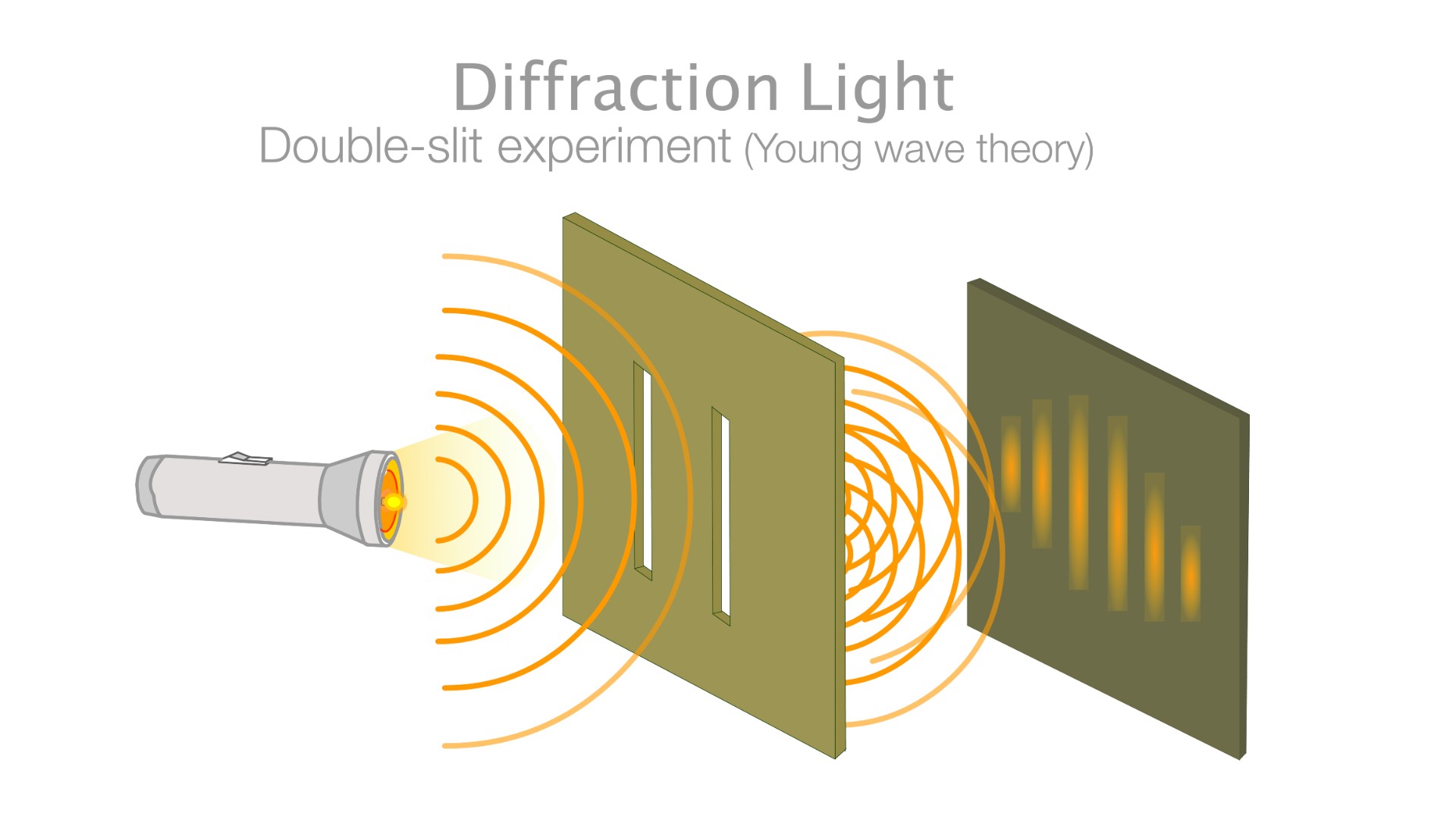
Particles can sometimes be waves and particles can be particles. This can be seen in the double-slit experiment, where particles are shot at a board with two slits cut into it, behind which sits a screen that lights up when an electron hits it. According to an article in Nature, if the electrons were particles, they would create two bright lines where they had hit the screen after passing through one or the other of the slit.
An interference pattern forms on the screen when the experiment is conducted. If the electrons are waves, with crests and troughs, that can interfere with one another, this pattern of dark and bright bands makes sense. An effect akin to a single electron interfering with itself can be seen when a single electron is shot through the slit.
In 1924, French physicist Louis de Broglie used the equations of Einstein's theory of special relativity to show that particles can exhibit wave-like characteristics and that waves can exhibit particle-like characteristics.
The internal structure of atoms was described using quantum mechanics by a physicist in the 1910s. By this point, it was known that an atom was made of a heavy, dense, positively charged nucleus surrounded by a swarm of tiny, light, negatively charged electrons. The electrons could only have certain preset distances around the nucleus. The atom can receive or emit radiation at specific energies by jumping from one orbit to another.
According to the American Physical Society, two scientists independently and using separate lines of mathematical thinking created a more complete quantum picture of the atom. In Germany, physicist Werner Heisenberg developedmatrix mechanics, and Austrian-Irish physicist Erwin SchrxF6;dinger developed a similar theory.
The earlier Bohr model of the atom was replaced by the Heisenberg-Schrdinger model. In the Heisenberg-Schrdinger model of the atom, electrons obey a wave function and occupy theirorbitals. According to an explanatory website from chemist Jim Clark, atomic orbitals have a variety of shapes, ranging from spheres to dumbbells to daisies.
Some of the early developers of quantum mechanics had reservations about the results of the thought experiment. While many of his students believed that quantum mechanics suggested that particles don't have well-defined properties until they are observed, Einstein and Schrdinger were not able to believe it because it would lead to ridiculous conclusions about the nature of reality. The life or death of a cat would depend on the random flip of a quantum particle, which would remain unseen until a box was opened. A real-world example that depended on the nature of a quantum particle but yielded a nonsensical result was intended to show the absurdity of the ideas.
The cat was alive and dead at the same time until the box was opened. No actual cat has ever been subjected to this experiment. Einstein believed that quantum mechanics was an incomplete theory and would eventually be superseded by one that accorded with ordinary experience.

The result of quantum mechanics could not be fully comprehended by Einstein and Schrödinger. Einstein, along with physicists Boris Podolsky and Nathan Rosen, showed in 1935 that two quantum particles can be set up so that their quantum states would always be correlated with one another. The particles are always about each other's properties. That means that measuring the state of one particle would tell you the state of its twin, no matter how far apart they were.
One of the most important aspects of quantum mechanics isanglement, which occurs in the real world all the time. The emergence of quantum computing is based on the phenomenon of quantum entanglement.
Physicists don't have a full explanation for all the observed particles and forces in the universe. quantum mechanics describes small and in substantial things, while Einstein describes large and massive things. Nobody knows how to make the two theories fit together.
A theory of quantum gravity would introduce gravity into quantum mechanics and explain everything from the subatomic to the supergalactic realm. No single theory has been able to fit all observations of objects in our universe, so there are a lot of proposals for how to do this. string theory, which claims that the most fundamental entities are tiny strings vibrating in many dimensions, has become less accepted by physicists since little evidence in its favor has been found. The idea of loop quantum gravity, in which both time and space come in tiny chunks, has been worked on by other researchers, but so far no one idea has managed to gain a major hold among the physics community.
The original article was written by Robert Coolman and was updated by Adam Mann.
Bow, June 19 A brief history of the light bulb. The history of the light-bulb can be found in Inside the Perimeter.
J. Clark was published in May. There are atomic orbitals.
Coolman, September 11. Classical mechanics? Live Science has classical mechanics.
O'Connor, J. J., and Robertson, E. F. There is a history of quantum mechanics.
Einstein was born in 1905. The production and transformation of light is a point of view. The Annals of Physics.
The favorite misunderstood pet of quantum mechanics is the cat. Live Science hasschrodingers-cat.
What is the theory of everything? Space.com has atheory of everything-definition.html.
C. Moskowitz was published on March 25. The largest molecule behaves like waves in the experiment. quantum-double-slit-experiment-largest-molecules.html
M. Schirber was published on July 9. What is the relationship between time and space? Live Science has a piece on what isrelativity.
The prize was awarded in the late 19th century. There are facts about Louis de Broglie.
E. Tretkoff was in February. February 1927 was the month in physics history when Heisenberg's uncertainty principle was used. The American Physical Society publishes a history of physics.
C. Wood was published on August 27. What is quantum gravity? quantum-gravity.html is available at Space.com.