Recent findings show how the immune responses that try to counter the onslaught of Hendra and Nipah viruses work. Multi-pronged tactics to prevent and treat these deadly illnesses are what the results point toward.
Today's research is reported in Science as a First Release peer-reviewed paper.
Bats native to certain parts of the world are capable of carrying both the Hendra and Nipah viruses. The henipaviruses can jump species and cause diseases. Brain inflammation and respiratory symptoms are caused by the viruses. People with either of these diseases have a 50% to 100% chance of dying.
There is a vaccine approved for use in horses that has entered a human clinical trial.
Horses can spread Hendra to their caretakers through saliva and mucus. Fifteen people who had a high-risk exposure have been given an experimental, but not yet approved, cross-reactive antibody. Emergency compassionate use guidelines were followed. The Phase 1 stage of testing has just been completed for this antibody, which is in a clinical trial in Australia. There are no approved vaccines or therapies for humans against henipaviruses, other than supportive care in the hope that the patient can overcome the virus.
New attempts to design life-saving preventatives and treatments became even more urgent after a new strain of Hendra was discovered. Over the past two decades, there have been many cases of the Nipah virus in Bangladesh. The disease has been seen in India and the Philippines. People and Pteropus bats in Africa have been found to have Henipaviruses. 2 billion people live in parts of the world where henipaviruses could be a threat.
The senior author of the latest henipaviruses paper in Science is a professor at the University of Washington School of Medicine and a Howard Hughes Medical Investigator. He studies bat immunity to many dangerous viruses and conducts research on the function and structure of theinfecting machinery. His lab is researching the interaction of the two families of viruses.
The lead author is a graduate student. Christopher Broder's lab collaborated on research at the Uniformed Services University and the Henry M. Jackson Foundation for the advancement of military medicine.
The researchers said that the viruses enter into cells through attachment and fusion. The key targets are the glycoproteins.
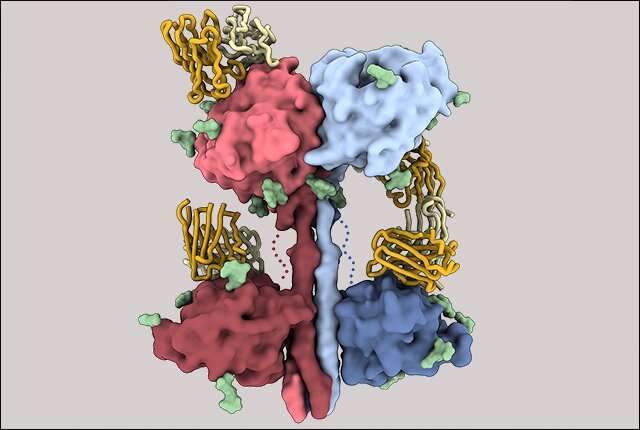
The scientists were able to determine the structure of a critical component of the Nipah virus through the use of cryoelectron microscopy. They observed that a mixture of antibodies work better together to disarm viruses. There were similar effects in the set of antibodies. The combination of forces helped keep the escape from happening.
vital information was provided by examining the antibody response in laboratory animals that were inoculated with the Nipah virus. The analysis showed which area of the virus was the most active in triggering an immune response.
The structure of the critical portion of henipaviruses responsible for eliciting antibody response was not known before this study. The lack of information made it difficult to understand immunity and improve the design of vaccine candidates.
Science may be closer to creating a template for building new and improved vaccines now that the researchers have uncovered the 3D organization.
An important part of the attachment structure has a neck and four heads. Only one of the four heads turns its binding site in the direction of the potential host cell, the other three turn away. The head domain can be re-oriented to engage with the host.
The scientists noted that the architecture of the paramyxoviruses is different from other paramyxoviruses. Most of the diseases they cause are transmitted on respiratory droplets. They include diseases that have recently crossed from animals to humans.
The scientists looked at two animals that had been immunized with the glycoprotein that was found in the Hendra virus. A potent, diverse neutralizing antibody response occurred. The head domain was found to be the main target of the neutralization, even though the full tetramer was used. The response narrowed on the binding area.
The researchers noted that the findings provide a blueprints for engineering next-generation vaccine candidates with improved stability and immunogenicity. They anticipate a design approach similar to that used for newer computer- engineered respiratory syncytial virus candidates. The body would be presented with a mosaic of head antigens on a multivalent display. Making large supplies of vaccine simpler is possible if you use only the head domain.
The title of the March 3 Science paper is "Architecture and antigenicity of the Nipah virus attachment glycoprotein."
More information: Zhaoqian Wang et al, Architecture and antigenicity of the Nipah virus attachment glycoprotein, Science (2022). DOI: 10.1126/science.abm5561. www.science.org/doi/10.1126/science.abm5561 Journal information: Science Citation: Henipavirus glycoprotein structure guides therapy, vaccine (2022, March 4) retrieved 4 March 2022 from https://phys.org/news/2022-03-henipavirus-glycoprotein-therapy-vaccine.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.