The researchers from Kanazawa University describe novel scanning cell microscopy measurements to determine the sites of photochemical activity in titanium dioxide nanotubes.
The power of light can be harnessed to produce other types of energy. One application of this technology is to produce hydrogen from water in a process called photoelectrochemical water splitting, which is a renewable source of energy.
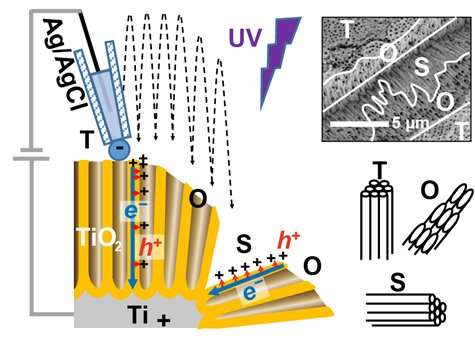
The hole and electron are separated when irradiated with light with the energy higher than a threshold. The PEC reactions are initiated by the flow of charge. Titanium dioxide is a widely used material for this purpose. The distribution of charge flow on the surface of TiO 2 tubes is not clear. A group of people in Japan and Europe have used an innovative technique to identify this distribution.
Oxygen release was measured to measure the success of PEC water splitting. The production was also characterized by lead added to the cell, so lead particles were deposited on the walls and top of the TiO 2 nanotubes, suggesting similar reactivity at both of these sites. The research team used SECCM to clarify the photoreactivity on both sites.
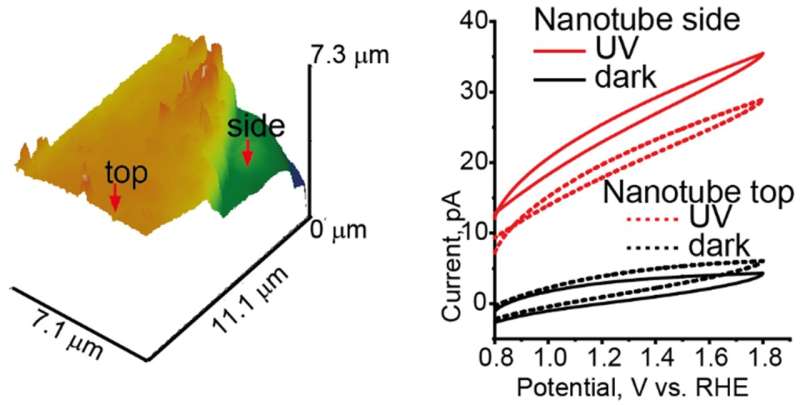
SECCM uses a narrow pointed pipette probe to measure electrical changes. The probe is filled with a conducting fluid which is located between two charged electrodes. The sample surface is connected to the second electrode that is immersed into the electrolyte. The reactivity on the walls and the top were measured parallel to the length of the tubes. The free electrons move along the length of the nanotubes towards the positive electrode. There are pockets of positive charge that can move when the electrons delocalize and jump to a conducting band. The electrical activity was similar along the walls and the top of the nanotubes. Since activity at the top could be attributed to the movement of electrons, it was suggested that holes were moving closer to the walls. PbO 2 depositions were found on the walls of the tubes because of the flow along a direction that was orthogonal to the tube length.
The team says that this information could be used to establish further correlations between the photocurrent and the microstructure of 1D nanostructures. Understanding spatial patterns of activity is important to design efficient and cost-effective photovoltaic cells. A combination of oxide deposition and SECCM might be the most sensitive tool for identifying active sites.
More information: Marina V. Makarova et al, Direct Electrochemical Visualization of the Orthogonal Charge Separation in Anatase Nanotube Photoanodes for Water Splitting, ACS Catalysis (2022). DOI: 10.1021/acscatal.1c04910 Journal information: ACS Catalysis Citation: Charge separation imaging on the surfaces of titanium dioxide photoelectrocatalytic nanotubes (2022, February 21) retrieved 21 February 2022 from https://phys.org/news/2022-02-imaging-surfaces-titanium-dioxide-photoelectrocatalytic.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.