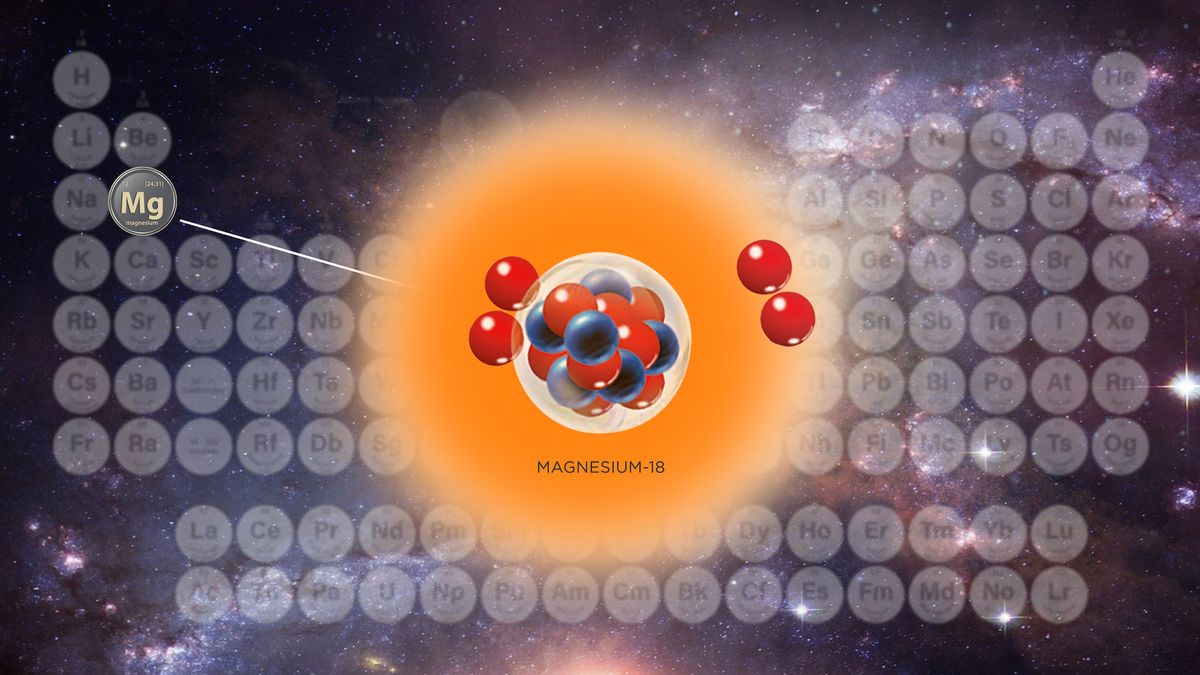
The lightest form of magnesium, which has just six neutrons in its atomic nuclei, was created inside a giant atom smasher.
The researchers expect their discovery to help scientists better understand how atoms are constructed. The limits of the models that scientists use to figure out how atoms work can be defined by the exotic isotopes.
Kyle Brown, a chemist at the Facility for Rare isotope Beams at Michigan State University, said that by testing the models in making them better and better we can learn how things work where we can't measure them. We're measuring the things we can to predict the things we can't.
The discovery of magnesium-18 won't fill all the gaps in scientific knowledge, but it will help refine the theories that scientists have developed to explain them. A summary of the research shows that the team's measurement of the radioactive decay's products give new insights into the binding energies of electrons.
The atomic nucleus is made of atoms.
Pure magnesium is a soft gray metal with 12 protons in its nucleus, which indicates it has a positive charge. Students in chemistry classes often see the white light from a burning magnesium strip.
The fusion reactions of aging stars cause magnesium to be found on Earth, because they seed the clouds that formed our solar system. Magnesium is abundant in the Earth's crust and it has an important chemical role in many biological and industrial compounds.
The most common stable isotope of magnesium has a neutral charge in each nucleus, giving it an atomic mass of 24. It's called magnesium 24.
The researchers accelerated a beam of magnesium 24 nuclei to half the speed of light inside the National Superconducting Cyclotron Laboratory at Michigan State University. The magnesium nuclei were fired at a metal foil.
The researchers were able to pick out a soup of lighter magnesium isotopes from the collision, which had an unstable magnesium-20.
The magnesium-20 nuclei were fired at a beryllium target about 100 feet (30 meters) away.
One of the products of the collision was magnesium-18, a light and rare magnesium isotope with just six neutrons and 12 protons in its nucleus.
A rare isotope.
Most atomic nuclei cancloak themselves with electrons from their environment and become atoms of other types, which can combine with other types to make chemical compounds.
The magnesium-18 isotope is very unstable and very short-lived, with a half-life of less than one-sextonth.
It disappears quickly for a nucleus of magnesium-18 to even have the chance to be a "naked" nucleus.
The magnesium-18 is so short-lived that it never leaves the beryllium target, and so the researchers deduced its presence from the telltale products of its decay: stray protons and oxygen-14.
Brown said that this was a team effort. It's not every day that people discover a new isotope.
Every year, scientists discover more and more of the 118 common elements.
"We're adding drops to a bucket, but they are important drops," Brown said. The whole team can put their names on this one. I tell my parents that I helped discover this nucleus.
The article about the discovery was published in the journal Physical Review Letters. Washington University in St. Louis and Peking University in China were involved.
Live Science published the original article.
