Malaria continues to plague parts of the globe. In 2019, it claimed the lives of more than 400,000 people, most of whom were children and young adults. It is even worse than COVID-19 in some areas of Africa. The coronavirus pandemic has seriously disrupted prevention and treatment efforts.
To make matters worse, it appears that a new strain, Plasmodium falciparum (the primary parasite that causes the disease), is now able to avoid the common ways we detect it.
Sindew Feleke, an immunologist at the Ethiopian Public Health Institute, has revealed that nearly 10% of malaria cases in Ethiopia are not being detected due to one or more of the mutations that allow the parasite to evade rapid diagnostic tests (RDT).
This mutated strain could also be able to hide from testing kits, giving it an advantage that can spread easily.
Jonathan Parr, University of North Carolina infectious disease researcher, stated that false-negative results are common at multiple locations and could lead to misdiagnosis or even death from malaria. "This is a problem for malaria control efforts, and a reminder of the fact that pathogens can adapt to survive."
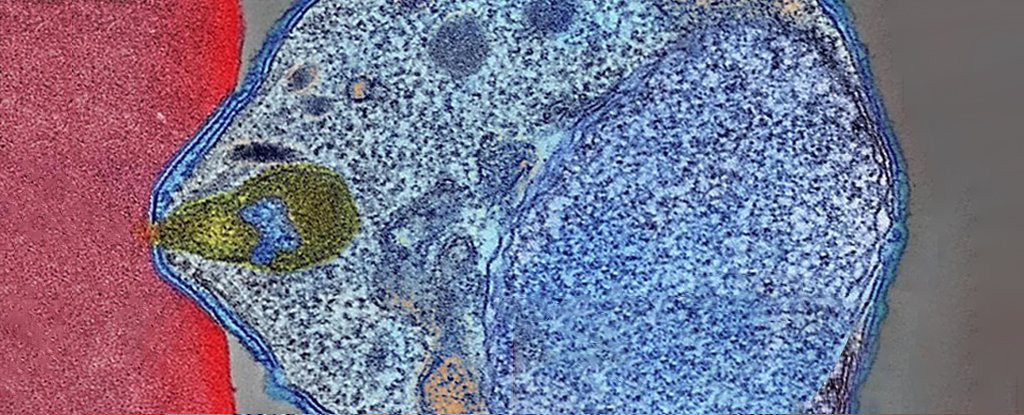
P. falciparum, the most deadly parasite responsible for malaria in humans, is the most prevalent. The parasite is spread by mosquitoes and infests red blood cells of humans to clone. The parasite eventually bursts from the blood cells, causing severe symptoms such as fever and bloody diarrhea. The majority of treatment for the disease revolves around decreasing human contact with blood-sucking insects.
Ethiopia, Africa's second-largest country, has made great strides in fighting malaria over the past decade thanks to rapid diagnostic testing. The most popular rapid test detects parasite-released antigens into the bloodstream. It is sold in large numbers, with around 345 millions annually. The test is triggered primarily by histidine-rich protein 2, (HRP2) but can also be triggered using the closely related HRP3.
Some mutations in P. falciparum (pfhrp2 or pfhrp23), have resulted in the deletion of the genetic instructions that code for the proteins.
Feleke and his team examined blood samples from over 12,500 Ethiopians living near Eritrea and Sudan and found that these genetic variants were responsible for false-negative results in just under 10% of the tests. This is twice the WHO minimum criteria to trigger a change in a national diagnostic strategy.
Parr explained, "We also discovered signs that RDT-based treatment and testing are driving a recent increase in pfhrp2 mutation prevalence, which allows parasites to escape detection."
Although the mutations were random in nature, the inadvertent treatment allows parasites with deletions in either one or both genes to thrive and spread.
For evidence of evolutionary pressure, the researchers mapped the sequences surrounding the deletions. The researchers discovered that pfhrp2 probably spread quickly from a single point of origin. 30 of 31 strains formed a cluster.
However, pfhrp3 is more recent, and can be found in samples as far back as 2013. There are also a variety of deletion patterns that suggest it may have had multiple origins.
Other testing options are less straightforward. RDTs that detect other parasite molecules have not been as successful.
The team points out that they missed people who were asymptomatic because they did not sample enough sites to get a full picture of the malaria strains. However, evidence from Somalia, Djibouti and Sudan suggests that P. falciparum may be already infiltrating the Horn of Africa.
The team stated in their paper that "new tools are required to support surveillance [gene] deletions and determine their true prevalence, and understand the forces impacting the evolution and spread of their spread."
These deletions can also be found in South America where RDT isn't common. Feleke and his colleagues believe they may confer an evolutionary advantage. It is possible that pfhrp2 also does this. Some evidence suggests that pfhrp2 may be involved in inflammation in severe malaria.
Researchers explain that people infected with pfhrp2/3 deleted parasites might have a less severe illness and be less likely to seek treatment. This increases the risk of onward transmission.
However, they cannot rule out the possibility of the mutations in the surrounding genes causing the parasite's fitness to grow. P. falciparum invades red blood cells through one of its flanking genes, EBL-1.
Parr stated that surveillance across the Horn of Africa is urgently required, as well as alternative diagnostic methods for malaria in affected areas.
Nature Microbiology published this research.
