DiA Imaging Analysis, an Israeli-based AI tech company that analyzes ultrasound scans using deep learning and machinelearning, has raised $14 million in Series B funding.
The growth round will be three years since DiA's last raise. It includes new investors Alchimia Ventures and Downing Ventures as well as ICON Fund, Philips, and XTX Ventures. Existing investors include CE Ventures and Connecticut Innovations. Mindset Ventures and Dr Shmuel Cabilly are also part of the group. It has raised $25M so far.
This latest financing will be used to expand its product range, pursue new partnerships with PACS/Healthcare IT companies and resellers as well as continue building its presence in three regional markets.
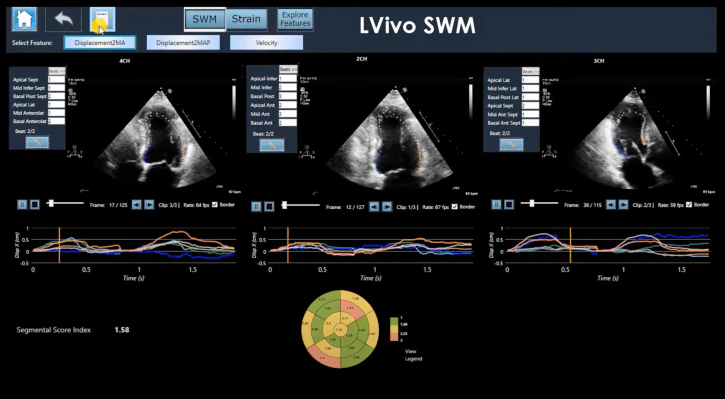
The AI-powered support software is sold by the healthtech company to healthcare professionals and clinicians to capture and analyze ultrasound imagery. This process, which can be done manually, requires human knowledge to interpret scan data. DiA claims that its AI technology removes the subjectivity from manual and visual estimations currently being done.
It has also trained AIs to evaluate ultrasound imagery in order to automatically identify key details and abnormalities. The company offers a variety of products that address different clinical needs associated with ultrasound analysis. These include software for measuring and analyzing aspects such as ejection fraction, right ventricle size, and function, and aiding with the detection of coronary disease.
The product also leverages ultrasound data to automatically measure bladder volume.
DiA claims that its AI software mimics the human eye's ability to detect borders and identify motion. It is a leap over subjective human analysis and promises speed and efficiency.
Hila Goldman, co-founder and CEO of our software tools, said that they are a support tool for clinicians who need to acquire the right image and interpret ultrasound data.
DiA's AI-based analysis can be found in around 20 markets, including North America, Europe and China. In China, it says that a partner has been approved for the use of the software in their own devices. The company is currently deploying a go to market strategy that includes working with channel partners such as GE and Philips which offer the software as an option on their ultrasound and PACS systems.
According to Goldman-Aslan at the moment, approximately 3,000+ users have access.
Our technology is cross-platform and vendor neutral. It can be used with any ultrasound device, or healthcare IT system. You can see that we have over 10 partnerships with both healthcare IT/PACS and device companies. She says that there is no other startup in the space with these capabilities, commercial momentum, or many FDA/CE AI solutions. She adds: We have seven FDA/CE approved solutions for abdominal and cardiac areas, and more are on their way.
The data-set it has been trained on will determine how well an AI performs. In the healthcare sector, efficacy is especially important as any bias in the data-set could result in a flawed model that misdiagnoses/under/overestimates disease risk in patient groups not represented in the training data.
Goldman-Aslan answered TechCrunch's questions about how the AIs were trained to spot key details in ultrasound images. He said: We have access hundreds of thousands of ultrasound images through many medical facilities so we can move quickly from one area to the next.
She explained that we collect data from a variety of devices and populations with different pathologies.
The phrase Garbage in Garbage Out is the best. She also explained that the key to avoiding garbage entering is to not bring it in. Our data is tagged and classified using several doctors and technicians who are each experts with years of experience.
A strong rejection system is also in place to reject images taken incorrectly. This is how we get rid of the subjectivity that comes with how data was acquired.
It is worth noting, however, that FDA clearances obtained from DiA are 510 (k) Class II approvals. Goldman-Aslan confirmed that it has not applied for Premarket Approval (PMA), and does not intend to.
For many medical devices, the 510(k route is used widely to obtain approval. It has been criticised for being a lightweight process and does not require the same level of scrutiny that the PMA process.
It is important to note that regulations of rapidly-developing AI technologies often lag behind their application. This is especially true as they move into healthcare, where there are huge promises but also serious risks. There is still a gap between what device manufacturers promise and how much regulatory oversight they actually receive.
The CE scheme, which establishes safety, health and environmental standards for devices in the European Union, can only require manufacturers to declare conformity without independent verification. However, some medical devices may require independent evaluations of conformity under this scheme. It is not a strict regime to regulate the safety of new technologies such as AI.
The EU is currently working to introduce an additional layer for conformity assessments, specifically for AI applications deemed high-risk under the forthcoming Artificial Intelligence Act.
The AIA would require additional regulations for healthcare use cases, such as DiAs AI-based Ultrasound Analysis. The EU co-legislators are currently discussing the proposal on the table. A dedicated regulatory regime for AI-related risks is still years away from being in force in the region.
