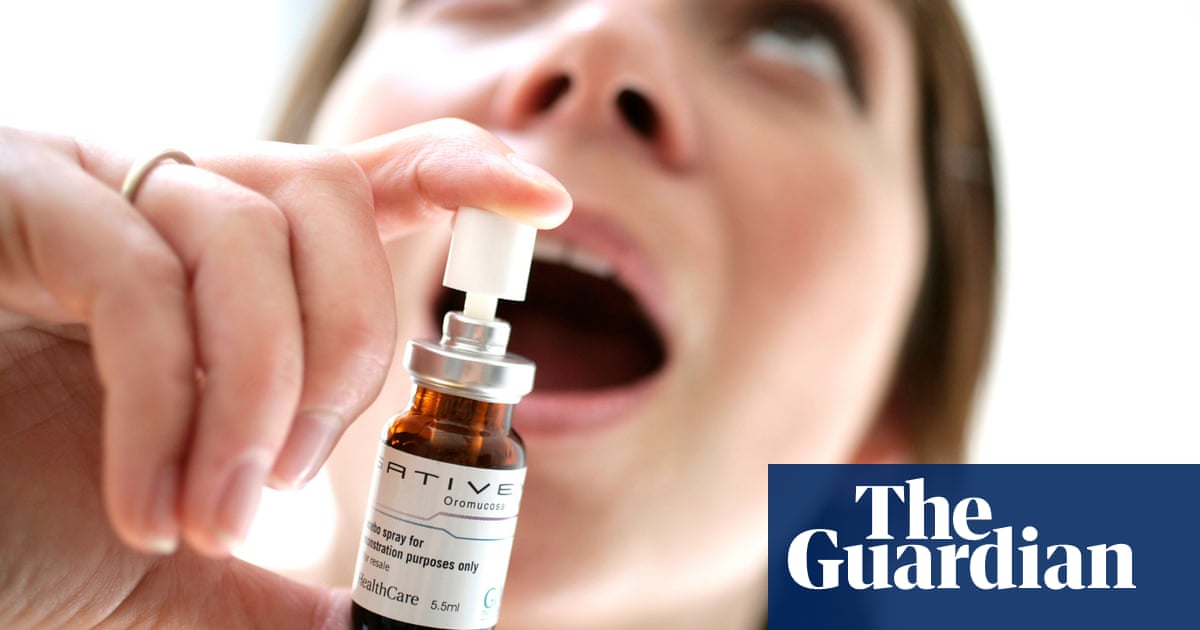
The NHS and cancer charities are currently investigating whether cannabis-based mouth sprays can be used to treat brain tumors and prolong the lives of patients.Patients with a recurrent brain tumor called a "glioblastoma" will be offered the drug Sativex by doctors in the UK. The medication is used in conjunction with chemotherapy medication temozolomide as part of a clinical trial to try and kill cancerous cells.It will be the world's first such study.Glioblastoma, an aggressive, hard-to-treat brain tumor, almost always returns despite doctors using radiotherapy, chemotherapy and surgery to treat it. Patients with recurrent glioblastoma live for only 12-18 months.This is the most common form of brain cancer, with approximately 2,200 people being diagnosed each year in England.Patients with multiple sclerosis who have not seen improvement despite treatment can be given Sativex to treat spasticity. It is one of three currently available cannabis-based medicines in the NHS.Sativex is thought to kill glioblastoma tumor cells. It may also be effective when combined with temozolomide chemotherapy. This may allow patients to live longer. Susan Short, a Leeds University professor of neuro-oncology and clinical oncology, is the principal investigator in the study. She said that this is what the study aims to test.The Brain Tumour Charity will fund the trial by recruiting 232 patients from at least 15 hospitals across the UK, including specialist cancer centers. The chemotherapy drug and the placebo will be administered to the remaining third of the patients.Sativex contains equal amounts of two cannabinoids: the psychoactive substance Delta-9-tetrahydrocannabinol (THC), which gives users a high, and cannabidiol (CBD), which can help reduce pain, inflammation and anxiety without inducing any psychoactive effects.The interim chief executive of Brain Tumour Charitys, Dr David Jenkinson, stated that we hope that this trial will open the door to a long-awaited lifeline that could give glioblastoma victims precious extra time to live and create memories with loved ones.We are aware that there is a lot of interest in the topic of cannabinoids as glioblastomas treatment, so we were excited to see this first-ever trial in the UK.This trial is a continuation of a previous study, namely a phase 1 trial that examined the safety of Sativex and temozolomide in combination. It involved 27 patients. The Aristocrat trial, a three-year study, will examine both the safety and the impact of the regimen on patients' outcomes, as well as how long they live.Jenkinson said that the early-stage results were very promising. Now, we look forward to learning whether Sativex could be added to chemotherapy to extend life and improve quality of life. This would be a significant step in our ability to cure this deadly disease.Short stated that the initial research suggested that Sativex could offer some people an extra life. Participants who received Sativex lived longer than those who received a placebo a year later.The results showed that the combination was safe, but some patients experienced side effects such as dizziness, fatigue, and sickness.This study did not test whether Sativex was more effective in terms of survival. She said that the study did show that Sativex patients did better than expected, and were more successful than patients who received chemotherapy.Although the Brain Tumour Charity intends to continue with the trial, it stressed that this was dependent on the outcome of an appeal to cover the 450,000 costs. After losing 25% of its income in the Covid-19 pandemic, it has now suspended its regular programme of research grants.Cancer Research UK's Birmingham university clinical trials unit is coordinating the new study. Professor Pam Kearns, director of the unit, stated that it is crucial that such trials, which examine the potential role of cannabis and the chemicals in it to treat cancer, be conducted.
