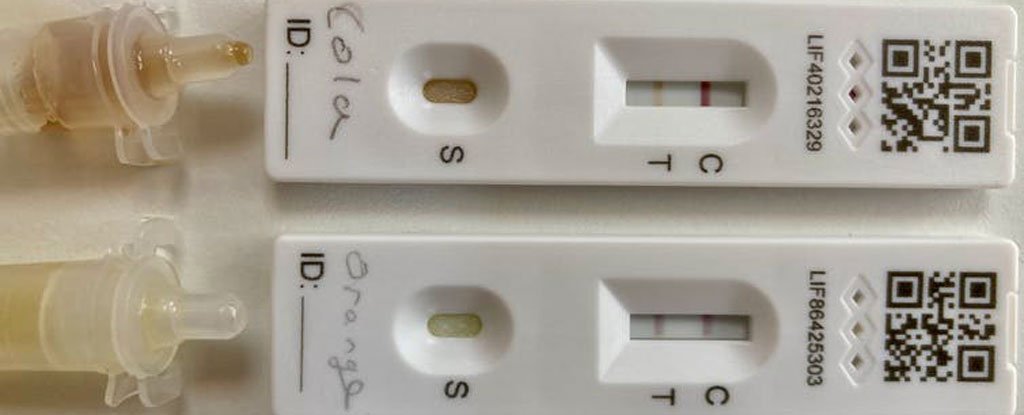
Children will always find clever ways to cheat school. The latest trick is to use soft drinks to create a positive COVID-19 LFT.How can fruit juices, cola and devious children fool the tests? Is there a way for you to distinguish a fake positive from a true one? This is what I tried to discover.First, I decided to verify the claims. I opened bottles of orange juice and cola, and then dropped a few drops onto my LFTs. Two lines appeared on each test a few seconds later. This supposedly indicated the presence of COVID-19.Understanding how these tests work is worth it. You will find a small strip of paper-like material called nitrocellulose and a small red pad under the plastic case below the T-line in an LFT device.The antibodies that bind the COVID-19 virus are found in the red pad. These antibodies can also be attached to tiny gold nanoparticles, which make it possible to see the location of the antibodies on the device.Before you can test the sample, mix it with a liquid buffer solution. This ensures that the pH of the sample remains at the optimum level before you apply it to the strip.Two COVID-19 home tests revealed fake positives from orange juice and cola. (Mark Lorch).The fluid absorbs the nitrocellulose strip, and then picks up gold and antibodies. If the virus is present, these antibodies also bind to it. There are additional antibodies that can bind to the virus further up the strip, near the T (for testing).These antibodies cannot move freely because they are bound to the nitrocellulose. These antibodies grab the virus as well, passing the second set of antibody red-labeled with gold.The virus is bound to both sets antibodies, leaving everything, even the gold, immobilized at a line near the T on the device. This indicates a positive test.The virus-bound gold antibodies don't attach to the virus and continue to climb the strip until they encounter a third set. These antibodies are not intended to pick up COVID-19 but instead stick at the C (for Control) line. They trap any remaining gold particles without the need to transmit the virus.This last line indicates that the test was successful.AcideHow can a soft drinks cause a red T line to appear?One possibility is that the drinks may contain an anti-viral substance that the antibodies recognize as well as bind to. This is unlikely. This is why antibodies are used in such tests.The saliva and snot from your mouth and nose can contain all kinds of things. Your antibodies will ignore the protein and other viruses in this mixture. They won't react to soft drinks.It is possible that the drink is altering the function of the antibody. Although a variety of fluids have been tried to fool the tests (cola and fruit juice), all have one thing in commun: they are high-acidic.These beverages have a pH of 2.5 to 4. These conditions are quite harsh for antibodies. They have evolved to function largely in the bloodstream with its nearly neutral pH of 7.4.To ensure the proper function of the test the pH must be maintained. This is the job of the liquid buffer solution provided with the test.The buffer's critical role is illustrated by the fact that you can mix cola and the buffer, as demonstrated in this debunking Austrian politician’s claim that mass testing has no value.The buffer is not necessary because the antibody test results are completely exposed to the beverage's acidic pH. This has a profound effect on the structure and function of their antibodies.Antibodies are proteins made up of amino acid building blocks that are linked together to form long, straight chains. These chains can be folded into very specific structures. A protein's function can be dramatically affected by a slight change in its chains.These structures are maintained through a network that includes many thousands interactions between different parts of the protein. Negatively charged proteins will attract to positively charged ones, for example.Acidic conditions can make the protein more positively charged. The protein's delicate structure is disrupted and many of its interactions are lost. This causes antibodies to lose their sensitivity to virus.This might lead you to believe that acidic beverages would produce completely unreliable results. Denatured proteins can be sticky. All the perfectly designed interactions that would hold the protein together are now lost and in search of something to bind.The most likely explanation is that the T-line's immobilized antibodies stick to the gold particles, causing the famous cola-induced false positive result.Is it possible to detect a false positive? Like most proteins, antibodies are capable of refolding in favorable environments and regaining function.(Mark Lorch).Above: A COVID-19 Test with a false positive due to cola, and a COVID-19 Test that used cola after it had been washed with buffer.So I was able to wash a test that I had dripped with cola and buffer solution. The immobilized antibodies at T-line were able to regain normal function and release the gold particles. This revealed the true negative result.Children, I admire your creativity. But now that I have uncovered your tricksery, I suggest you use your cunning and devise a series of experiments to test my hypothesis. We can then publish your results in peer-reviewed journals.Mark Lorch, Professor of Science Communication and Chemistry at the University of HullThis article was republished by The Conversation under Creative Commons. You can read the original article.
