Subscribe to Journal
Get full journal access for 1 year
$199.00
only $3.90 per issue
All prices are NET prices.
VAT will be added later in the checkout.
Rent or Buy article
Get time limited or full article access on ReadCube.
All prices are NET prices.
Additional access options:
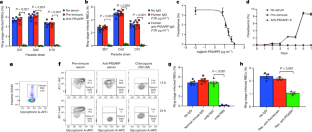
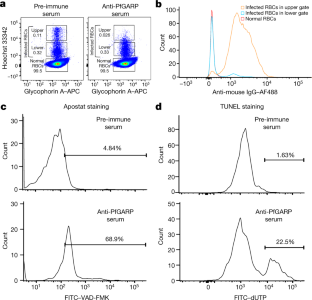
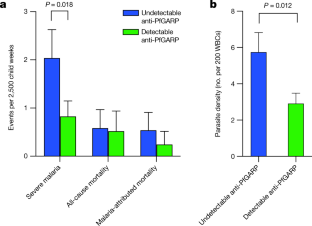
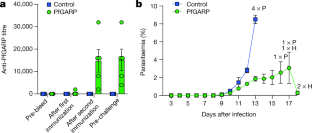
Source Data for Extended Data Figs. 5 and 9 are provided with the paper. The DNA sequence for PfGARP is available in PlasmoDB ( www.plasmodb.org) under the gene ID PF3D7_0113000. All other relevant data are available within the manuscript and its Supplementary Information. We thank MOMS project staff for their efforts in collecting clinical data, processing samples and interpreting malarial blood smears, and we thank the study participants and their families. This work was supported by grants from the US NIH (R01-AI076353, R01-AI127699 and R01-AI110699) and an internal Rhode Island Hospital Research Pilot Award grant to J.D.K.; grants from the US NIH (R01-AI52059) and the Bill & Melinda Gates Foundation (grant no. 1364) to P.E.D.; the Intramural Research Program of the NIH National Institute of Allergy and Infectious Diseases (NIAID), NIH grant R01-AI092120 and Florida Atlantic University start-up funds to A.V.O.; NIH grant R37-AI50234 to D.A.F.; and NIH grants R01-AI145941 and R01-AI102907 to J.D.D. We also acknowledge research core services provided by the Rhode Island Hospital imaging core (G. Hovanesian), the Leduc Bioimaging Facility (G. Williams) and core services supported by the COBRE Center for Cancer Research Development (P20GM103421). The Thermo Apreo Volume Scope scanning electron microscope that was used for the serial block-face imaging was purchased with a high-end instrumentation grant from the Office of the Director at the NIH (S10 OD023461). L.L., S. O.-G., M.F. and P.E.D. are supported by the Intramural Research Program of the NIAID. Author notes Present address: Department of Microbiology and Molecular Genetics, University of California, Davis, Davis, CA, USA These authors contributed equally: Michal Fried, Patrick E. Duffy, Jonathan D. Kurtis J.D.K., D.K.R., M.F. and P.E.D. conceived and supervised the study. J.D.K., P.E.D., M.F., J.F.F., S.P., C.B.M., D.A.F., D.K.R. and A.D.M. analysed the data and/or drafted the text. D.K.R., A.D.M., A. Jnawali, J.Z., A. Jha, G.C.-K., B.S., C.E.N., N.H., S.P.-T. and L.B. contributed to parasite killing assays. D.K.R., J.Z., A.D.M., G.J., D.A.F., A.M. and N.F.G. contributed to imaging studies. P.E.D., M.F., J.D.K. and E.K. contributed to field-based data collection. P.E.D., M.F., S.P., J.F.F., A.V.O., O.C., J.D.K. and J.M. contributed to epidemiological analyses. R.M.R. and J.D.D. contributed to genetic modification of parasites. N.P., D.W., B.L.M. and Y.K.T. contributed to mRNA-based vaccine design and production. D.K.R., L.L., S.O.-G., M.F., P.E.D. and J.D.K. contributed to non-human primate studies. Correspondence to Jonathan D. Kurtis. The work presented in this manuscript has been submitted in partial support of patent no. US10,213,502 B2 (filed 26 May 2017) on the use of PfGARP as a vaccine, on which the authors J.D.K., D.K.R., J.F.F., M.F. and P.E.D. are named inventors. The remaining authors declare no competing interests. Peer review information Nature thanks Peter Preiser and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. a, SDS-PAGE gel of purified rPfGARP-A (250 ng). b, c, We analysed rPfGARP-A, as well as extracts prepared from uninfected RBCsand 3D7 trophozoite-infected RBCs by western blot. b, Blots were probed with mouse polyclonal anti-PfGARP-A serum (generated by plasmid immunization) and with anti-MSP2 (rabbit polyclonal serum), which were detected with anti-mouse IgG (red) and anti-rabbit IgG (green). c, Blots were probed with pre-immune mouse serum and anti-MSP2 (rabbit polyclonal serum), detected with anti-mouse IgG (red) and anti-rabbit IgG (green). Data in a- c are representative of five independent experiments. d, GIAs performed on parasites collected from our field site in Muheza, Tanzania, after short-term adaptation to the culture. Assays were performed using polyclonal anti-PfGARP-A antibodies generated by immunizing mice with recombinant protein. Ring-stage malaria parasites from adults (NIH 00710 and NIH 00918) or children (NIH 408551 and NIH 4122821) were cultured in the presence of anti-PfGARP mouse serum at a 1:10 dilution. Negative controls were no antiserum (blue) and pre-immune mouse serum (red). Parasites were cultured for 48 h at 37 °C and ring-stage and early-trophozoite-stage parasites were counted by microscopy. Data are mean ± s.e.m. of five biologically independent replicates. P values were calculated by two-sided non-parametric Mann-Whitney U-test. Data are representative of five independent experiments. e- h, 3D7 P. falciparum parasites were synchronized to the ring stage and plated at 5% parasitaemia in the presence of pre-immune ( e, g) and anti-rPfGARP-A ( f, h) mouse serum at a 1:10 dilution. Parasites were cultured for 24 h ( e, f) or 48 h ( g, h), stained with Giemsa and photographed by light microscopy. Images in e- h are representative of three independent experiments. i- l, Purification of anti-PfGARP antibodies from human ( i, j) and mouse ( k, l) serum. Serum was affinity-purified using PfGARP-A coupled to sepharose beads. The specificity of the purified anti-PfGARP antibodies was determined by western blot on extracts prepared from unsynchronized 3D7 parasite-infected RBCs. In all panels: lane 1, infected RBCs extracted in RIPA buffer; lane 2, uninfected RBCs extracted in RIPA buffer. Blots in i, j were probed with anti-PfGARP purified from serum that was pooled from adults living in a holoendemic area of Tanzania (2 μg ml −1) ( i), or with immunoglobulin from malaria-naive adults (2 μg ml −1) ( j). Blots in k, l were probed with anti-PfGARP purified from serum that was prepared from PfGARP-A-immunized mice (10 μg ml −1) ( k), or immunoglobulin from malaria-naive mice (10 μg ml −1). Blots in i- l are representative of two biologically independent experiments. a, The kinetics of binding between the recombinant monoclonal antibody mAb7899 and PfGARP-A were measured at two antibody concentrations. For each concentration, two biologically independent replicates were performed. The experiment was performed twice and the data were pooled. The dashed lines represent the 95% CI. Error bars represent s.d. The formula is a linear regression. C, concentration of biotinylated recombinant mAb7899; MFI, median fluorescence intensity; V, initial velocity of binding. b, Epitope mapping of recombinant mAb7899. We printed a custom 15-mer-peptide microarray containing 264 different peptides that spanned the PfGARP-A sequence (amino acids 410-673). The peptides overlapped by a single amino acid and were printed in duplicate, framed by haemagglutinin (HA) control peptides. The array was probed with recombinant mAb7899 (red) and anti-HA (green) and imaged on an LI-COR Odyssey. c, RBCs infected with ring-stage 3D7 parasites were cultured in the presence of medium alone, recombinant anti-PfGARP monoclonal antibody or recombinant anti-fluorescein. Parasites were cultured for 48 h at 37 °C and the levels of lactate were measured in the culture supernatant. Data are mean ± s.e.m. of four biologically independent replicates. The P value was calculated by non-parametric two-sided Mann-Whitney U-test. d, RBCs infected with ring-stage 3D7, D10 or Dd2 parasites were cultured in the presence of medium alone or recombinant anti-PfGARP Fab antibody (1 mg ml −1) for 48 h at 37 °C, and ring-stage or early-trophozoite-stage parasites were counted by microscopy. Data are mean ± s.e.m. of three biologically independent replicates. P values were calculated by non-parametric two-sided Mann-Whitney U-test. Data are representative of two independent experiments. a, Targeting strategy for creating 3D7-PfGARP KD parasites. b, Immunoblot analysis of 3D7-PfGARP KD. Parasites were sorbitol-synchronized at the ring stage, incubated with or without anhydrotetracycline (ATc) for 20 h and blots were probed with anti-V5. The expected molecular weight of the PfGARP V5-tagged protein is 124 kDa; however, its apparent mobility is 165 kDa owing to its acidic composition. Lane 1, uninfected RBCs; lane 2, RBCs infected with 3D7-PfGARP KD parasites and cultured without anhydrotetracycline; lane 3, RBCs infected with 3D7-PfGARP KD parasites and cultured with anhydrotetracycline. c, d, Expression of PfGARP on the surface of human RBCs infected with 3D7-PfGARP KD parasites. Ring-stage 3D7-PfGARP KD parasites were cultured to the trophozoite stage in the absence ( c) or presence ( d) of anhydrotetracycline. Expression of PfGARP on the surface of fixed but not permeabilized infected human RBCs was determined by flow cytometry, using monoclonal anti-V5 as the primary and anti-mouse IgG-Alexa Fluor 488 as the secondary antibody. Infected RBCs were gated and identified as described in Fig. 1e. e, Growth curves for 3D7-PfGARP KD parasites. Ring-stage parasites were cultured with or without anhydrotetracycline. Parasitaemia was measured by microscopy. Data are mean ± s.e.m. of three biologically independent replicates. f, GIAs using 10% anti-rPfGARP-A serum or pre-immune serum on 3D7-PfGARP KD parasites cultured with or without anhydrotetracycline. Data are mean ± s.e.m. of three biologically independent replicates. The P value was calculated by non-parametric two-sided Mann-Whitney U-test. Data are representative of three independent experiments ( b, d, e, f) or five independent experiments ( c). a, Targeting strategy for creating 3D7-PfGARP KO parasites, b, Immunoblot analysis of 3D7-PfGARP KO. Trophozoite-stage 3D7 wild-type or 3D7-PfGARP KO parasites were probed with anti-PfGARP, and with anti-histone H3 as a loading control. Lane 1, RBCs infected with 3D7-PfGARP KO parasites; lane 2, RBCs infected with 3D7 wild-type parasites. c, d, Expression of PfGARP on the surface of human RBCs (fixed but not permeabilized) infected with 3D7-PfGARP KO parasites. Ring-stage stage 3D7 wild-type or 3D7-PfGARP KO parasites were cultured to the trophozoite stage. Expression of PfGARP was determined by flow cytometry, using anti-PfGARP as the primary and anti-mouse IgG-Alexa Fluor 488 as the secondary antibody. Infected RBCs were gated and identified as described in Fig. 1e. e, Growth curves for 3D7-PfGARP KO parasites. Ring-stage 3D7 wild-type or 3D7-PfGARP KO parasites were plated at 0.5% parasitaemia and cultured for 6 days. Parasitaemia was measured by microscopy. Data are mean ± s.e.m. of three biologically independent replicates. f, Ring-stage 3D7 wild-type or 3D7-PfGARP KO parasites with targeted deletion of PfGARP were cultured at a 1:10 dilution in the presence of anti-PfGARP-A mouse serum that was generated by immunizing mice with PfGARP-A-mRNA LNPs. Negative controls were no antiserum (blue) and pre-immune mouse serum (red). Parasites were cultured for 48 h at 37 °C and ring-stage and early-trophozoite-stage parasites were counted by microscopy. Data are mean ± s.e.m. of six biologically independent replicates. P values were calculated by non-parametric two-tailed Mann-Whitney U-test. Data are representative of two independent experiments. a, Uninfected and infected RBCs were probed with mouse anti-PfGARP prepared by DNA vaccination (green) and with rabbit anti-PfMSP4 (red) and counterstained with DAPI to label parasite nuclei. PfGARP is detected on the membranes of RBCs infected with early-, mid- and late-trophozoite-stage parasites and does not colocalize with PfMSP4 (which localizes to the parasite membrane). DIC, differential interference contrast microscopy. Scale bars, 5 μm. b, Trophozoite-infected RBCs do not label when probed with pre-immune mouse serum. Scale bars, 5 μm. c, d, Non-permeabilized, unfixed trophozoite-infected RBCs were incubated with polyclonal anti-rPfGARP ( c) or control mouse serum ( d), probed with anti-mouse IgG labelled with 10-nm gold particles, fixed, embedded and visualized by transmission electron microscopy. PfGARP localized to the outer leaflet of trophozoite-infected RBCs. Right, higher-magnification views of the boxed areas on the left. Images are representative of five ( a, b) or two ( c, d) biologically independent experiments. Source Data a, Uninfected and infected RBCs were probed with rabbit anti-glycophorin A (anti-GYPA; green) and mouse anti-PfGARP prepared by DNA vaccination (red) and counterstained with DAPI to label parasite nuclei. PfGARP is detected only in trophozoite-infected RBCs and colocalizes with human glycophorin A on the RBC membrane. Scale bars, 5 μm. b, Neither early- nor late-trophozoite-stage-infected RBCs label when probed with pre-immune mouse serum. Scale bars, 5 μm. Images are representative of three independent experiments ( a, b). a, 3D7-PfGARP KD parasites, in which PfGARP is tagged with the V5 epitope, were grown in the presence of anhydrotetracycline to induce the expression of PfGARP, probed with rabbit anti-glycophorin A (green) and mouse anti-V5 (red) antibodies and counterstained with DAPI to label parasite nuclei. V5-tagged PfGARP colocalizes with glycophorin A to the exofacial surface of trophozoite-infected, non-permeabilized RBCs. Scale bars, 5 μm. b, 3D7-PfGARP KD parasites were grown in the presence of anhydrotetracycline to induce the expression of PfGARP. RBCs infected with trophozoite-stage 3D7-PfGARP KD parasites were fixed and incubated with anti-V5 mouse IgG ( b) or buffer ( c) and probed with anti-mouse IgG labelled with 10-nm gold particles. V5-tagged PfGARP localized to the outer leaflet of 3D7-PfGARP KD-infected, non-permeabilized RBCs. Right, higher-magnification views of the boxed areas on the left. d, 3D7-PfGARP KD parasites were grown in the presence of anhydrotetracycline to induce the expression of PfGARP, probed with anti-PfCRT (green) and anti-V5 (red) antibodies and counterstained with DAPI to label parasite nuclei. PfGARP does not colocalize with PfCRT to the food vacuole in the majority of trophozoite-infected RBCs. Images are representative of three ( a), two ( b, c) or five ( d) biologically independent experiments. 3D7 parasites were synchronized to the ring stage and plated at 5% parasitaemia in the presence of pre-immune ( a) or anti-rPfGARP-A ( b) mouse serum at a 1:10 dilution. Parasites were cultured for 24 h and processed for transmission electron microscopy. Data are representative of all trophozoites observed in three biologically independent experiments ( a, b). c, Ring-stage 3D7 parasites were treated with pre-immune or anti-PfGARP serum (1:10) or 1 μM chloroquine for 24 h, followed by staining with DAPI and Fluo-4 AM. Data are representative of two biologically independent experiments. a, Individual parasitaemia data from the monkey trial presented in Fig. 4. Aotus monkeys vaccinated with PfGARP-A-mRNA LNPs ( n = 5) and control monkeys vaccinated with poly(C)-RNA LNPs ( n = 4) were challenged intravenously with 1 × 10 4 P. falciparum-infected RBCs and parasitaemia was followed daily. H indicates treatment for low haemoglobin; P indicates treatment for high parasitaemia. b, Animals were injected subcutaneously either with 50 μg of rPfGARP-A emulsified in 100 μl Ribi ( n = 4 monkeys) or with Ribi alone (negative control; n = 5 monkeys) at weeks 0, 3 and 6, and PfGARP-A-specific IgG titres were determined. Data are mean ± s.e.m. c, Vaccinated Aotus monkeys were challenged intravenously with 10 4 P. falciparum FVO strain-infected RBCs on day 63 and parasitaemia was followed daily. Data are mean ± s.e.m. Control monkeys had significantly higher parasitaemia on days 7-12 than monkeys immunized with PfGARP. On day 11, the final day with complete follow-up of all monkeys, control monkeys had 3.5-fold-higher parasitaemia than PfGARP-vaccinated monkeys. Four control monkeys met pre-specified criteria for drug treatment on day 11 and the remaining control monkey met these criteria on day 12. On day 11, one PfGARP-vaccinated monkey underwent drug treatment despite not meeting the pre-specified criteria. * P < 0.05, ** P < 0.01 in two-sided t-tests without adjustment for multiple comparisons. d, Parasitaemia data from the individual monkeys in c. Source Data a, Schematic depicting the binding sites for the peptide-specific antibodies. b- e, Immunoblot of rPfGARP-A (lane 1), recombinant full-length PfGARP (lane 2) and an extract of trophozoite-infected RBCs (lane 3) probed with antibodies raised against amino acids 504-522 of PfGARP ( b) or pre-immune serum ( c), or with antibodies raised against amino acids 31-48 of PfGARP ( d) or pre-immune serum ( e). Only antibodies raised against amino acids 504-522 recognized native PfGARP in trophozoite-infected RBCs, whereas antibodies raised against amino acids 31-48 only recognized the full-length recombinant PfGARP, confirming that the PEXEL motif is cleaved during the processing of native PfGARP. Pxl, PEXEL motif; SP, signal peptide. Data are representative of three biologically independent experiments. P. falciparum 3D7 parasites were synchronized to the ring stage and incubated with Left Panel) pre-immune mouse sera, or Right Panel) anti-PfGARP-A (1:10 dilution) for 30 hours and processed, embedded, and imaged for Serial Block Face-Scanning Electron Microscopy. The erythrocyte membrane is rendered in red, the parasitophorous vacuole in blue, the food vacuole in purple and hemozoin in yellow. Results representative of 2 biologically independent experiments. This file describes protein processing of PfGARP, with associated references. This file contains uncropped page gel and blots with molecular size markers and gating strategy for flow cytometry data. This file contains Supplementary Tables 1-3 P. falciparum 3D7 parasites were synchronized to the ring stage and incubated with Left Panel) pre-immune mouse sera, or Right Panel) anti-PfGARP-A (1:10 dilution) for 30 hours and processed, embedded, and imaged for Serial Block Face-Scanning Electron Microscopy. The erythrocyte membrane is rendered in red, the parasitophorous vacuole in blue, the food vacuole in purple and hemozoin in yellow. Results representative of 2 biologically independent experiments. Raj, D.K., Das Mohapatra, A., Jnawali, A. et al. Anti-PfGARP activates programmed cell death of parasites and reduces severe malaria. Nature (2020). https://doi.org/10.1038/s41586-020-2220-1 Nature Reviews Drug Discovery (2020)Data availability
Acknowledgements
Author information
t
Affiliations
t
t
t
t
t
t
t
t
t
t
Contributions
Corresponding author
Ethics declarations
Competing interests
Additional information
Extended data figures and tables
Supplementary information
About this article
Cite this article
Further reading