lecanemab, the second treatment from Biogen and its Japanese partner, was granted accelerated approval by the FDA on Friday.
Results from a clinical trial published in November showed that lecanemab slows cognitive decline in people with mild impairment due to Alzheimer's disease, but the treatment carries risks of brain swelling and bleeding.
The name Lecanemab will be used.
If the drug is expected to help patients with more serious conditions than what is currently available, the agency can quickly approve it. The drug was developed by the two companies together.
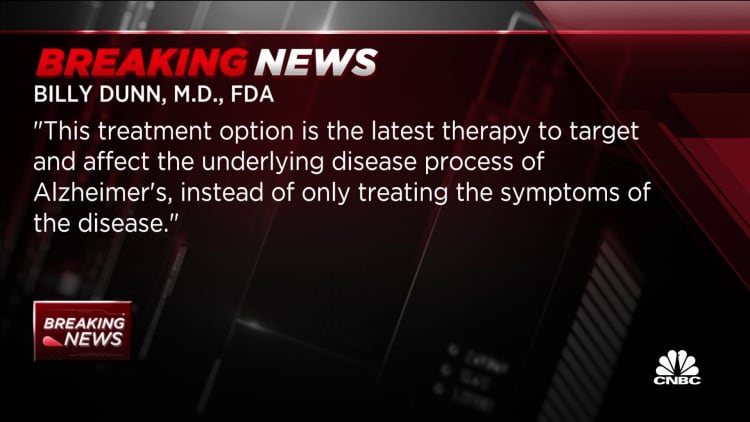
The director of the FDA's neuroscience division stated thatAlzheimer's disease is incapacitating the lives of those who suffer from it and has devastating effects on their loved ones. The latest therapy to target and affect the underlying disease process of Alzheimer's is the treatment option.
The decision on lecanameb comes after Congress issued a report about how the FDA handled the approval of Aduhelm. According to the report, the approval of that treatment in 2021, which did not show a clear clinical benefit, wasrife with irregularity.
The FDA must take swift action to make sure that its processes for reviewing future Alzheimer's disease treatments do not lead to the same doubts about the integrity of FDA's review.
Lecanemab is a drug that is used to treat Alzheimer's disease. Every two weeks, the patient's body weight is taken into account when determining the amount of the antibody given.
lecanemab was approved by the FDA due to the reduction of amyloid plaque observed in clinical trial participants who received the treatment. The placebo arm did not reduce amyloid plaque.
The cognitive decline in people who received lecanemab was 27% slower than in people who didn't. The study was paid for by two companies.
The cognitive decline was measured using a system called the clinical dementia rating, which is an 18 point scale with a higher score indicating a greater level of impairment. It takes into account cognitive functions such as memory and judgement.
Alzheimer's disease progressed 1.21 points on average in the group that received lecanemab compared with 1.66 points in the group that did not receive the treatment.
Half of the people who received lecanemab did not have early Alzheimer's.
The treatment carries risks and may slow cognitive decline.
The group that received lecanemab had a higher incidence of brain swelling than the group that didn't. Most of the cases were mild to moderate in severity and did not cause symptoms.
Some patients who received lecanemab had more serious brain swelling with symptoms such as headaches and confusion.
The group that did not take the treatment had a higher rate of brain bleeding. Deficiency was the most common symptom.
There were more serious adverse events for people who received lecanemab than for people who did not.
The efficacy and safety of lecanemab need to be determined in patients with early Alzheimer's disease.
There will be a warning about a risk of swelling and bleeding in the prescription information for lecanemab.
A research letter published in the New England Journal of Medicine states that the death of a clinical trial participant in the Chicago area could be linked to lecanemab.
The 65-year-old had a stroke after their third lecanemab injection. There was extensive bleeding in the brain after the patient's stroke. There was no bleeding before the stroke.
t- PA is a medication used to break apart blood clot that causes strokes. The doctors who wrote the research letter said that extensive brain bleeding would be an unusual side effect of this medication alone.
In a response letter, researchers argued that the blood clot medication appeared to be the cause of the patient's death, with the first symptoms occurring 8 minutes after they received the blood-clot buster.