
Inexpensive iron salts can be used to make essential precursors for drugs and other chemicals.
In the production of drugs and agricultural chemicals, they have refined the process. Iron salts, along with other processes, make it affordable and eco-friendly.
Two graduate students in the lab of a Rice synthetic chemist and their co-lead author report in Nature Communications that illuminating their reagents with visible light allows them to form diazides in more gentle conditions.
There are two amine groups in the molecule that can be functionalized and they can react with other molecules. They can be the basis of a lot of useful compounds.
In a recent study, West and his group added two functional groups to a single alkene molecule that contained at least one carbon-carbon double bond.
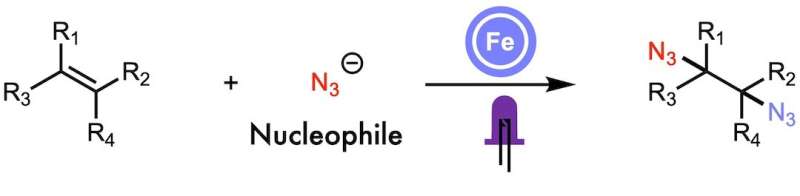
The technique, along with iron-mediated ligand-to-metal charge transfer, came in handy.
"It only uses two reagents, iron nitrate and TMS azide, which every synthetic lab will have," said West, an assistant professor of chemistry. You mix them together and shine light on them. The majority of pharmaceutical labs have lights. They will remove things from the shelf.
West said it was inspired by biology and included the enzymes in our own bodies. The potential of that one step in the last study was exciting to us.
Now that we know how that works, we can combine it with new steps to make something different. Nature appreciated a long time ago that this can be useful.
When the reagents and solution are illuminated in ambient conditions, there are two different types of transfer. The lab was able to maximize the process by running the solution through a looping tube and lighting it.

In the part where you shine the light, the reaction occurs. We can process more batches if we speed up or slow down the flow.
It's easy to dump the salts in the flask and shine a light on it, but if you want to make a lot, or make it better, flow works really well.
It will be helpful for labs that want an easy way to make this kind of product if they don't have the time to fine tune and fight with other methods.
David Nemoto Jr. and Xiaowei Chen co-authored the study.
The photochemical diazidation of alkenes is enabled by ligand-to-metal charge transfer.
Journal information: Nature Communications