While conducting an otherwise straightforward investigation into the assembly mechanism of calcium-phosphate clusters, researchers at UC Santa Barbara and NYU made a surprising discovery.
The recent uncovered behavior has implications for understanding the role of phosphate species in biocatalysis, cellular energy balance and the formation of biomaterials. The findings are published in a journal.
Songi Han is a chemistry professor at the University of California, Santa Barbara. Four oxygen atoms surround a single phosphorus atom. Han said that it's in the blood and the Serum. It's on our genes in every Biologist's buffer. She said that it's a structural part of our bones.
When bound with calcium, phosphates form small clusters on their way towards forming mineral deposits. Matthew Helgeson at UCSB and Alexej Jerschow at NYU were preparing to study and characterize in hopes of uncovering quantum behaviors in the proposed clusters. In order to set up the control experiments, the researchers had to use nuclear magnetic resonance andcryo- TEM.
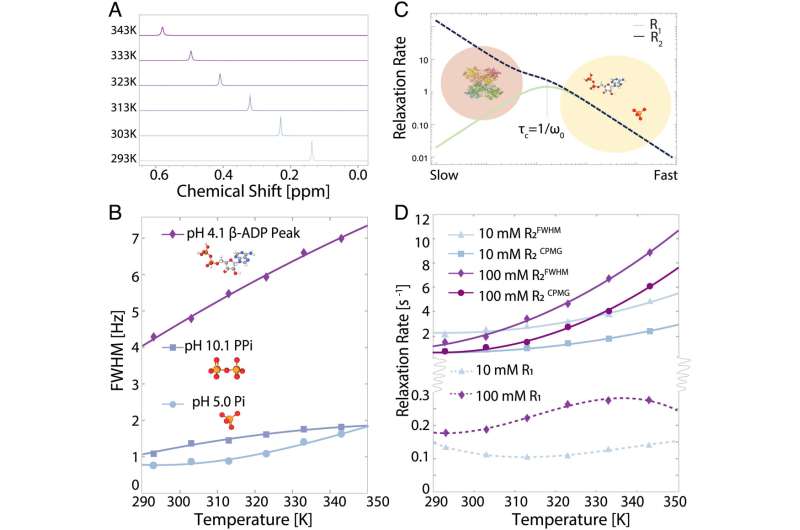
The results of the data collected by the UCSB and NYU students were not in line with expectations. Han said that the spectrum for 31P is supposed to narrow with increasing temperatures.
She said that higher temperatures cause the molecule to tumble faster. The average out of the anisotropic interactions is based on the relative orientations of the small molecule. A narrowing of resonances would be achieved.
She said they were expecting a simple signal with a peak that narrowed with higher temperatures. We were surprised to find that the spectrum that we measured was not what we expected.
The team was set on a new path after this unexpected result. Is the conclusion after a year of eliminating one hypothesis? cluster formation under a wide range of biological conditions was the reason why they had not been observed before. The broadening of the signal instead of a sharp peak was suggested by the measurements.
According to co-lead author Mesopotamia Nowotarski, as the temperature increased, the number of these assembled states increased as well.
She said that the dehydrating of thephosphates allowed them to come closer together. Most of thephosphates in solution cling to the water molecule that forms a protective water coat around them. It is assumed that this hydrated state is what behaves in biological systems.
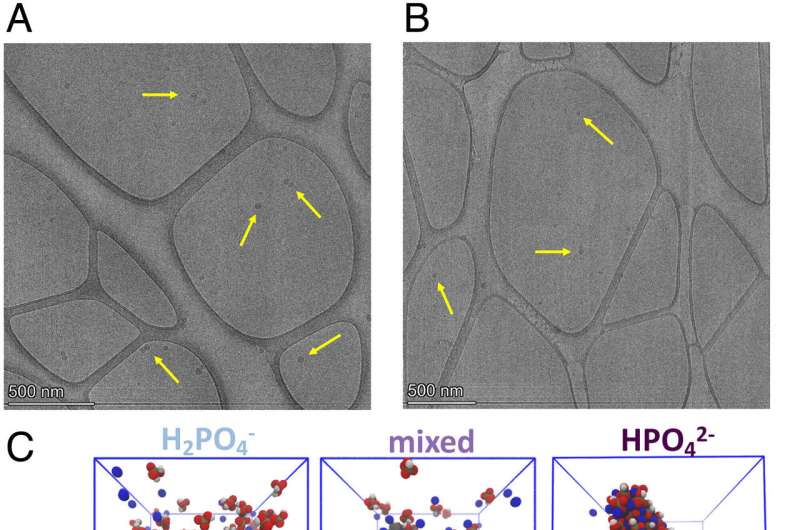
They were able to stick to each other because of their water shields. The concept was confirmed by NMR experiments that probed the phosphate water shell, as well as analysis of cryo-TEM images to identify the existence of clusters.
The researchers say that the hydration shells and dynamicphosphate assembly have important implications. Matthew Helgeson said thatphosphate is used in biological systems to store and consume energy.
The discovery suggests that small 'bills' of currency can exchange with larger denominations, which may have different interactions with biochemical processes.
Many biomolecular components contain groups that may form clusters. The discovery that these phosphates can spontaneously assemble skeletons might shed some light on other biological processes such as biomineralization.
Jiaqi Lu, one of the co-lead authors, said that they achieved quantitative analysis for the assemblies after testing a range of phosphates.
This once overlooked process could be significant in the realm of cell signaling, metabolism and disease processes such as Alzheimer's disease. The team has seen and studied the assembly behavior and is now looking into the effects of pH on the assembly.
Han said that it changes the way we think about the role of phosphate groups.
In the Proceedings of the National Academy of Sciences, Joshua S. Straub and his colleagues report on the formation of dark state assemblies in common solutions ofphosphates. 10.1073/pnas.2206765 120
Journal information: Proceedings of the National Academy of Sciences