Cell communication has been a fascination for researchers for a long time. The question of how cells use their sense is a mystery that has been solved by Professor Otger Camp and his colleagues. The paper has been published.
The surroundings are being tested.
In their paper, the researchers show how cells within a living embryo test their environment and what they see. Cells sense and respond to mechanical signals in a dish. "However, their microenvironment is quite different within an embryo and we did not know what mechanical cues they perceive in a living tissue."
The mechanical cures help cells make important decisions, such as whether or not to divide, move or even differentiate, the process by which stem cells turn into more specialized cells able to perform certain functions.
Stem cells on surfaces that are similar to bones become bone cells, while cells on surfaces that are similar to brain tissue become neurones. Researchers were able to create synthetic scaffolds to encourage stem cells to develop into desired outcomes as a result of the findings. Today's scaffolds are used in a wide range of applications.
The embryo came from a dish.
A dish is not the cell's home. Cells are not in contact with synthetic scaffolds in a flat dish but with complex living materials.
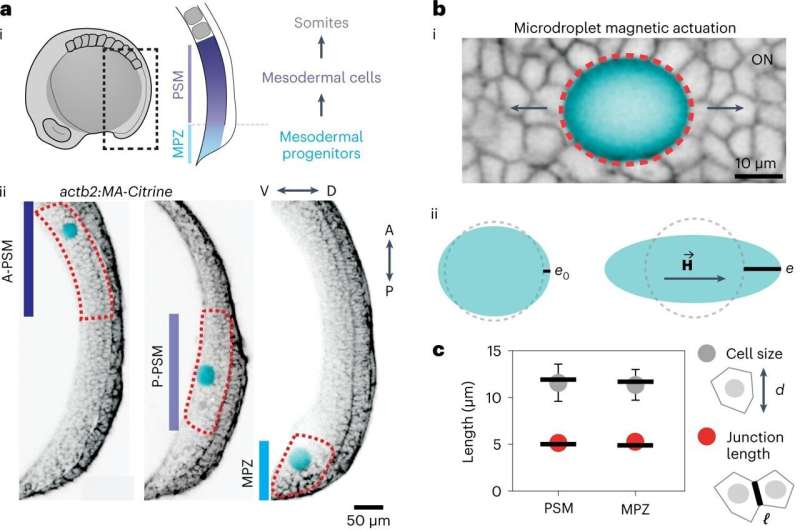
Over the last decade, Prof. Camps' research group discovered the mechanical cues that guide cells in an embryo. Using a unique technique developed in his lab, the researchers could probe the living tissue and find out what mechanical structures the cells sense.
Cells pushed and pulled on their environment. We measured how fast and strong they were.
They were able to mimic these tiny forces and measure the mechanical response of the cells by using a ferromagnetic oil droplet that they inserted between developing cells.
Cells and tissue architecture can be changed.
Camps and his research group described in a previous paper how the collective physical state of the cells is critical to their actions and how it is similar to an active foam.
The collective state of this "living foam" is what the cells are mechanically probing. At the moment, cells differentiate and decide to change their fate, there is a change in the material properties of the tissue that they perceive. He said that at the moment the cells within the tissue decide on their fate, the tissue becomes stiff.
In the future going forward.
The question of how the change in the cell state is caused is not yet proven in this study.
The mechanical characteristics of the structures that cells collectively build, such as tissues or organs, and the decisions they make individually, are dependent on the mechanics that cells sense in the tissue. The interplay is at the core of how nature builds organisms.
Implications for tissue engineering may be found in the findings from this study. Potential materials that mimic the foam-like characteristics of the embryo may allow researchers to create more robust and sophisticated synthetic tissues, organs and implants in the lab, with the appropriate geometries and mechanical characteristics.
There is more information about the mechanics of the cellular microenvironment.
Journal information: Nature Materials