Doctors don't wait until the patient's arteries are blocked to begin treatment for high cholesterol While the person is healthy, they prescribe cholesterol-lowering drugs.
Millions of baby boomers are at risk of Alzheimer's disease, and some of the world's biggest drug companies are trying to find a cure. Thousands of healthy adults will be tested on brain-plaque-relocating drugs in massive final-stage trials. The hope is that cognitive decline will be delayed or staved off before it starts.
The Centers for Disease Control and Prevention is where this information comes from.
There have been many failed attempts to get a treatment. Two years has passed since a new generation of anti-Alzheimer's agents began to show signs of slowing the disease's progress. When given to people who already show symptoms, compounds from Eli Lilly and Eisai slow cognitive decline by only 30%.
By the time people start to show signs of Alzheimer's, the damage to key brain areas may be extensive. Recent brain scans show that amyloid, which is the target of most of the new drugs, accumulates in the brain as long as two decades before dementia.
Eliminating amyloid before it causes damage will give patients a greater impact. If the concept pans out, healthy adults in their late 50s or early 60s would be able to take blood tests or specialized brain scans to look for the build up of amyloid ortau, two abnormal proteins. They would have to decide whether or not to use amyloid-lowering drugs if they were positive.
If you wait until people have symptoms, you may not be able to fully prevent the problems. It's important to remove the amyloid before people are impaired.
He knows a thing or two about Alzheimer's. Around 2008 she began to notice subtle symptoms in her father. She was told by the doctor that there was nothing wrong with him. After five years, he was diagnosed with Alzheimer's.
Results from the first big trial could come next year. The National Institute on Aging and Eli Lilly are co-sponsors of a trial that will test a drug on healthy older people. Researchers are using a higher dose of the drug in the prevention trial because it failed in three other trials. It is not as potent as newer amyloid-lowering drugs, but it avoids the brain swelling that can occur with newer drugs.
The more recent trial was sponsored by the National Institute on Aging and was started in 2020. It is giving lecanemab to healthy people as young as 55 with moderate to high amyloid levels. The goal is to start early. Lecanemab is being developed with a partner.
Eli Lilly's newer amyloid-lowering drug, donanemab, is under review by the FDA for accelerated approval as a treatment. The company thinks nine monthly doses may be enough to remove amyloid in healthy people.
A 51-year-old woman died of Alzheimer's disease after Alois Alzheimer noticed deposits in her brain. The correlation between high levels of amyloid and cognitive decline has become more apparent in recent years as researchers developed brain- Scan technologies that make it possible to measure amyloid levels in the brains of living people.
A 59-year-old costume historian in southern Maine joined the lecanemab prevention trial because she is terrified of getting Alzheimer's. All of her family members died from Alzheimer's. Before dementia struck, her mother was a high school language teacher who was proficient in five different languages.
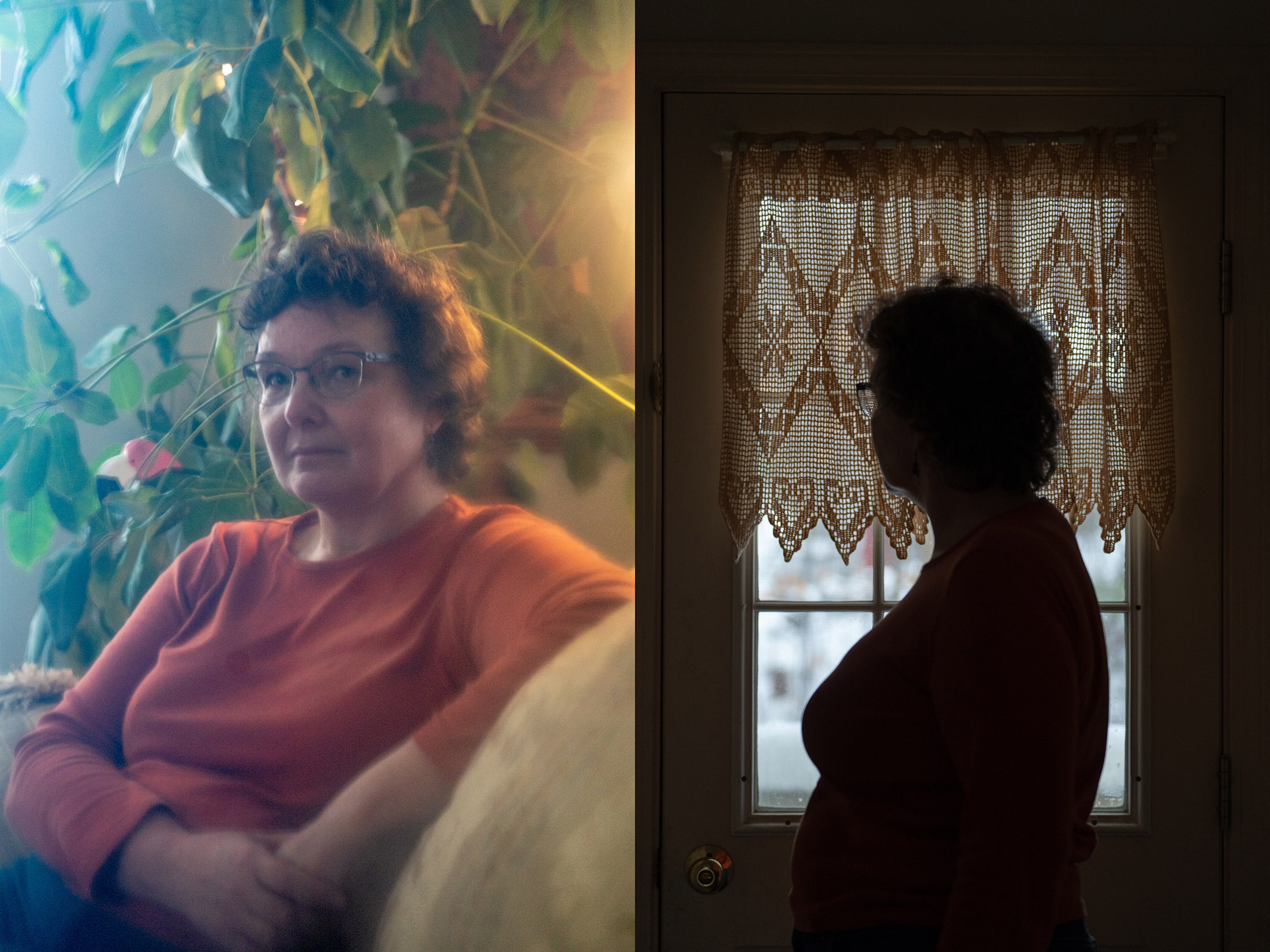
A year ago, after hearing about the lecanemab prevention trial, she got an amyloid scans and learned she had high levels. She goes to Harvard's Brigham and Women's Hospital in Boston every two weeks for trial-related treatments.
She doesn't know if she's getting the active drug, but being in the trial makes her feel less powerless. She says it would be worth it if it prevented her from losing her brain.

Brain scans show that amyloid builds up slowly for years before symptoms appear, promoting brain inflammation and eventually leading to cognitive decline. By the time the symptoms are obvious, removing a lot of theprotein does nothing. The lecanemab slowed the rate of cognitive decline in patients with early Alzheimer's even though it removed most of the brain amyloid. It is expected to lead to US approvals for the drug next year, and eventually billions of dollars in sales for the company. It's not clear how noticeable the change will be for most patients.
The lecanemab and solanezumab prevention trials are being conducted by the University of Southern California. He estimates that removing amyloid in healthy people on the verge of cognitive decline could delay the start of Alzheimer's by six years.
The lecanemab and solanezumab trials will look at the effects of amyloid on the brain. Eli Lilly wants to show the drug can prevent disease. More than 400 people will progress to mild cognitive impairment or dementia before the company takes action. The cases will be counted to see if the number of people who took the drug decreased.
Eli Lilly hopes that people in the donanemab arm will have less progression to symptoms.
Alzheimer's drug development is a huge bet for Big pharma. It raises questions about what the US can afford and whether the benefit is worth it. A quarter of older adults have amyloid in their brain. The current amyloid-lowering agents are expected to cost at least $25,000 a year. They don't know if they'll prevent disease. The more powerful drugs can cause brain swelling and brain bleeding.
The cognitive decline on Eli Lilly's donanemab was 32% slower than on a placebo, but they had a 39% rate of brain swelling or brain bleeding. The deaths of two people who took blood thinners while on lecanemab were not caused by the drug, according to the company.
Critics say the trials are too short and can detect subtle cognitive differences that aren't meaningful. Abandoning amyloid reduction research is not likely due to the public's fear of Alzheimer's.
According to the director of the neuroscience division at the National Institute on Aging, there could be millions of people who convert into Alzheimer's in the future. Something that could delay that could have a huge impact.
Next up is Living Better With Alzheimer's Thanks to a Village Square.