One hundred fifty years ago, Charles Darwin thought that life began in a pond. There, according to Darwin, chemical reactions and the occasional lightning strike might have led to the creation of chains of amino acids.
Researchers have been trying to figure out the chemical pathways that could have led from a pool of simple amino acids to a group of organisms. John Yin is a professor of chemical and biological engineering and a founding faculty member of the Wisconsin Institute for Discovery. Their work was published in the journal.
The Miller-Urey experiment was conducted in 1952 and involved the simulation of the conditions on the Earth. The researchers found that when zapped with electricity, the reaction produced amino acids, suggesting that the molecule was present on the planet.
Yin says that the building blocks of the human body are essential. It's long been a question of how we could get these things to form bonds and strings in a way that leads to a living cell. The chemistry involved tends to fail in the presence of water, so the question is difficult.
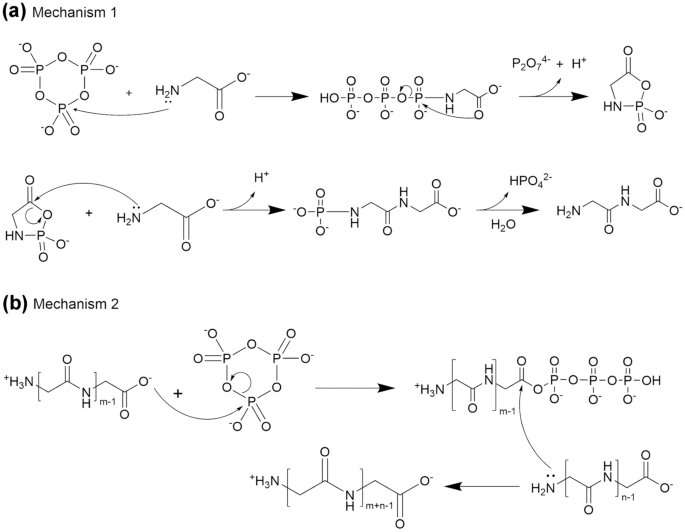
As a pool of water evaporated, Boigenzahn wondered if these amino acids could have come together during a period of environmental change. The short chains of amino acids could be formed in the presence of a chemical activator.
Boigenzahn created solutions of the two acids to study how they might form bonds during the drying process. Boigenzahn was able to see what happened to the amino acids over the course of a day.
The process was two stages. When the solution's pH was neutral, the two-molecule units called dimers, which are also produced protons, were created. As the second stage took place, the dimers began to bond together to form long chains called oligoglycine.
It's easy to imagine a scenario in which a volcanically warmed hot spring contains an activator that causes the formation of dimers. As the water evaporates and its chemistry changes, the dimers bond and form into long chains of amino acids.
Boigenzahn says that it doesn't have to be the same environment for all the reactions. They can happen in different environments if the reactions that are occurring help create an environment that is beneficial for the next steps.
It is1-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-65561-6556 Eventually, they could have begun to form their own enzymes, orProteins that act as catalysts for chemical reactions The beginning of metabolism could be set in motion by that.
It will be a long time before researchers figure out a path from Darwin's warm little pond to the beginning of life, according to Boigenzahn and Yin. It's important for chemical engineers to study prebiotic chemistry.
If you understand chemistry, you can create chemical systems that are able to adapt and evolve. The density of a computer chip can be thousands of times greater than the density ofDNA. If we could get systems that do this without being living cells, then you would start to think about all sorts of new functions and processes occurring at the molecule level.
There is more information about Glycine to Oligoglycine via sequential trimetaphosphate activation steps in drying environments.