A new structure has been found that helps single-celled organisms survive in the hot acid springs of the U.S.
The fact that life can survive in the boiling and highly acidic waters of the hot springs is amazing.
An international research team studied an acid-loving species of archaea to discover how this is possible.
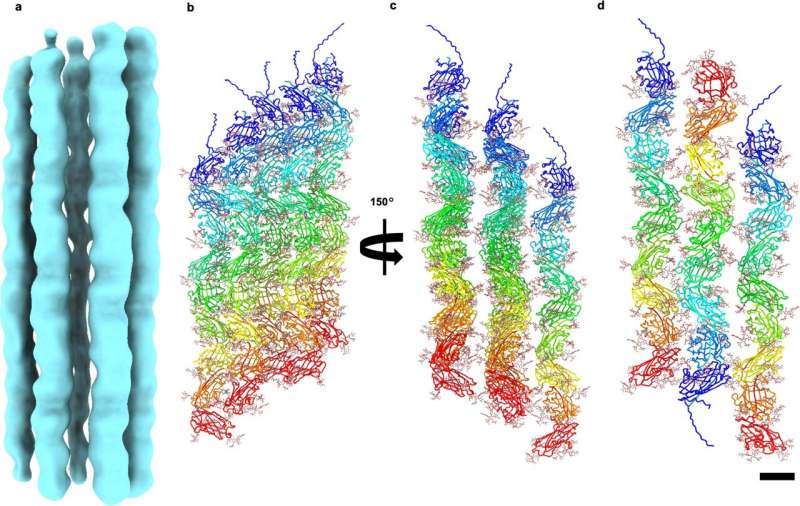
Using a cutting-edge technique called electron cryo-microscopy, they examined the hair-like proteins produced by the species, and found a previously unknownProtein that forms extremely stable chain-like fibers
According to the study, only special life forms can survive in the hot springs.
One of the most common archaeal species on Earth is S. acidocaldarius, which thrives in 80C and acidic pH values.
S. acidocaldarius creates toxic sulfuric acid through the oxidation of sulfur in the springs.
The team wanted to understand how the cells come together.
Understanding the structure of S. acidocaldarius' four different protein filaments could reveal how this species survives, as well as aid the development of strong, yet bio-degradable nanomaterials.
The structure of the thread was examined.
Special incubators were used to grow S. acidocaldarius in Germany.
We used a transmission electron microscope to image the threads after they were frozen.
A highly detailed three-dimensional image of the thread was created using sophisticated image analysis software.
The team was surprised by the discovery of a previously unseen class of archaeals.
The co-author of the study explained that the threads are made of tadpole-shaped proteins.
Each tadpole-shaped subunit inserts its tail the head of the next one along the chain.
Even under the most adverse conditions, the bonds allow the cells to join.
The structure of the archaeal thread is revealed in the study.
The structure of the archaeal thread is revealed in a study.
Journal information: Nature Communications