The structure of a bacterium has been exposed by researchers at the University of Alabama atBirmingham. High-resolution knowledge of structure is a key link betweenviral biology and potential therapeutic use of the virus to quellbacterial infections.
Bacteriophages or "phages" is a term used for viruses that cause illness. The team of researchers led by Terje Dokland and Asma Hatoum-Aslan at the University of Illinois have described atomic models for all or part of 11 different structuralProteins The study was published in a journal.
There is a group called the picoviruses. The range of its hosts is limited. Infections of indwelling medical devices are caused by this skin bacterium. Hatoum-Aslan is a phage Biologist at the University of Illinois.
With the emergence of antibiotic resistance, researchers are interested in the use of bacteriophages to treat infections. They have other characteristics that make them attractive candidates for therapeutic use.
It's possible to make custom-made phages using genetic manipulation if you know the structure of the virus and how it works.
According to Dokland, the structural basis for host specificity between the two infections is still poorly understood. With the present study, we have gained a better understanding of the structures and functions of the genes, paving the way for a more rational design of custom phages. There are critical features for the assembly of the virus.
Depending on the type of wall teichoic acid on the surface of different bacterial strains, staphylococcal phages can have a wide range of infections.
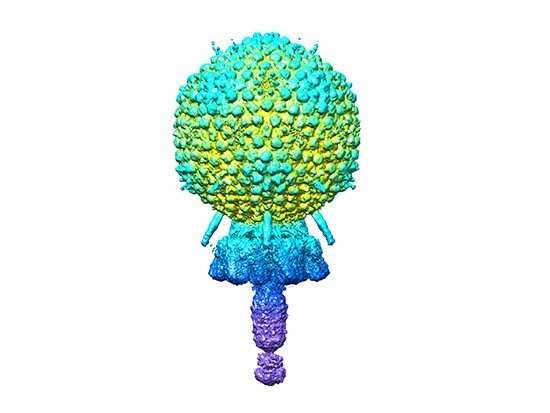
There is a capsid head that contains the viral genome. There is a short tail. The cell wall is broken by the tail. The genomes are injected through the tail. The portal from the cap to the tail is one of the segments of the tail.
Multiple copies of the same viruses particle are made from the same 11 different proteins. The capsid is made of more than 200 copies of two genes, while the other nine are made of less than two. The structure of two copies of a 12thProtein was predicted using the AlphaFold program, which is used to predict the structure of many genes.
The atomic models described by Dokland, Hatoum-Aslan, and co-first authors are shown in the picture. The researchers have described how the different parts of the capsid are made up, as well as how the different parts of the capsid interact with one another.
Light microscopes use a beam of electrons to illuminate an object, whereas electron microscopes use a beam of electrons to illuminate an object. The element of super-cold temperatures makes it useful for near-atomic structure resolution of larger and smaller samples.
New electron detectors have created a huge jump in resolution for cryo-electron microscopy over the last eight years. The "resolution revolution" is related to the use of cryo-electron microscopes.
The analysis began with more than 200 thousand images. The capsid, tail, distal tail and tail tip were reconstructed with images. The resolution ranged from 3.50 to.90 angstroms.
There is more information about the structure and host specificity of Staphylococcus epidermidis. There is a book titled "Sci Adv.ade 0.0459."
Journal information: Science Advances