The scientists at RIKEN used an off-the-shelf compound for the first time to create a self-healingpolymer. The strategy they used is promising for reducing the environmental impact of commercial polymers for a wide variety of applications.
The costs and burden on the environment would be reduced by the ability of the materials to heal themselves. Chemical reactions are the most common way to produce self-healing polymers. Self-healing mechanisms may not work in acidic and alkaline environments.
Material scientists would like to use simple synthesis processes to make polymers that self-heal under a wide range of conditions.
Polyolefins are the most widely used synthetic polymers in the world. Food packaging, clothing, automobiles and electronic and medical devices are just some of the things that use polyolefins. The lifetime, safety, and environmental impact of materials used in many applications could be improved by making self-healable polyolefins.
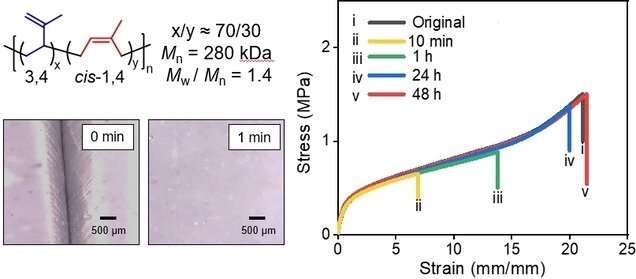
Hou and his co-workers have been able to make a synthetic equivalent of rubber latex that has a self-healing mechanism. The same building block as regular polyisoprene is used to make their self-healing polyisoprene.
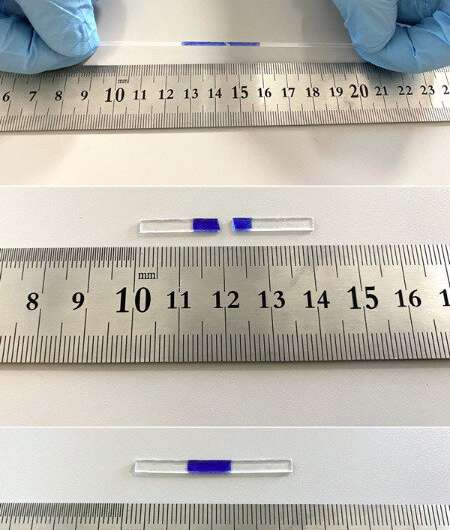
The team brought the two pieces of the block together at room temperature after cutting it in half. The sample could carry weights up to 2.5 kilomes.
They used a rare-earth catalyst to create a mixture of two different types of polyisoprene The other one was soft. The self-healing property could be achieved with a 3:1 ratio of the hard and soft material. The team believes that the network gives rise to self-healing because of the links between the hard microstructures.
Hou says the ultimate goal is to use easily available commodity monomers to produce tough self-healing polymers that are capable of spontaneously repairing when they sustain mechanical damage in real world environments. This work can help achieve this goal.
The work is in an international journal.
There is more information about Making Polyisoprene SelfHealable through Microstructure Regulation by Rare Earth Catalysts. There is a book called 10.1002/anie.
Journal information: Angewandte Chemie International Edition