The nucleus of an atom is formed by a combination of particles.
The number of protons and the number of neutrons in the nucleus can affect the isotopes of elements. Ordinary hydrogen has one protons and no neutrons, but the isotopes of hydrogen, deuterium and tritium have two protons and a single neutron.
Three small particles called quarks are held together by the Strong Force and are part of the nucleus. One up and two down quarks are contained in a neutron. The baryonic 'visible' matter in the universe is caused by particles made from three quarks.
The theory of everything is related.
RECOMMENDED VIDEOS FOR YOU...
After Ernest Rutherford discovered that atoms have a nucleus in 1911, and nine years later discovered that atomic nuclei are made, at least in part, by protons, the discovery of the neutron was made.
The idea of something else in an atom's nucleus came from the fact that the number of protons did not match the weight of the atom. An oxygen atom has 8 protons but has an atomic weight of 16 suggesting that it has other particles. Since atoms don't have an electric charge, they have to be neutral.
Scientists were using alpha particles to bombard a material made from the element beryllium with an alpha particle stream. The alpha particles produced particles that looked like they came from the beryllium atoms. When the mystery particles hit the target, they would knock the protons out of it. The mystery particles have to have the same mass as a protons. He won a prize for his discovery after proclaiming the particle to be the neutron.
They have no charge because of their neutral state. Their mass is almost identical to the mass of the protons.
Neutrons prefer to be on their own outside the nucleus. The binding energy of the Strong Force between them and protons in the nucleus keeps them stable, but when out on their own they become a protons, an electron and an antineutrino.
Einstein said that mass and energy are 888-609- 888-609- 888-609- 888-609- 888-609- A slight difference in the mass of a protons and a neutrons means that the latter has more mass than the former. When a neutron decays, it creates a pair of particles.
A variation of an element with more neutrons is known as an ide. At the top of the article, we showed the example of the hydrogen isotopes deuterium and tritium, which have 1 and 2 extra neutrons. Deuterium is a stable isotope. Some are unstable and radioactive. Tritium has a half-life of about 12 years, but other isotopes decay much more quickly, in a matter of minutes.
When inducing a chain reaction, nans are an essential tool. Nuclear fission takes place when unstable isotopes are absorbed by atomic nuclei and split into smaller daughter nuclei of other elements. When an extra neutron is absorbed, it becomes unstable and breaks apart, releasing energy.
The r-process is a mechanism used to create heavy elements in massive stars. The process of forging elements by stars was described in the famous B2FH paper by the Burbidges, Fowlers and Hoyle.

Nuclear fusion reactions can create elements of oxygen, nitrogen and carbon. The shells of heavier elements can be created all the way down to iron 56 in the star's core. At this point, the reactions require more energy to be put into them to fusion elements heavier than iron, so those reactions cease, energy production grinds to a halt and the core of the star collapses. It's possible to liberate a lot of free neutrons in a short period of time, because of the violent blast of a supernova.
In the supernova blast atomic nuclei are able to sweep up all the free neutrons before they all decay. The extra neutrons turn into protons when the nucleus is full of them. New heavy elements such as gold, Platinum and other precious metals can be created by adding protons to a nucleus. The gold in your jewelry came from a supernova.
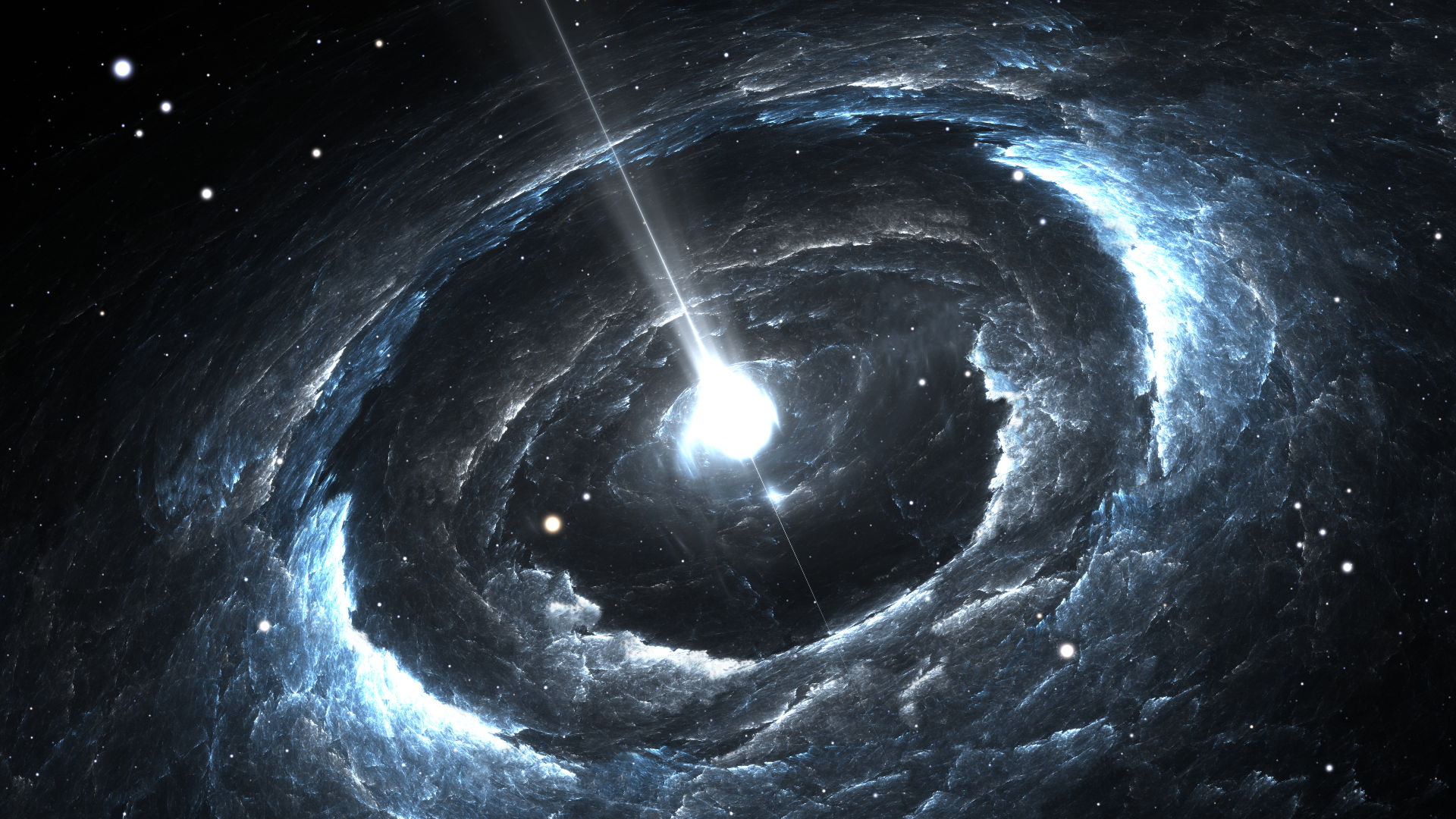
There are only a few places in the universe more extreme than neutron stars, and only in the most extreme conditions can they survive. These are objects that are mostly made of neutrons.
After a star's core collapses and explodes as a supernova, it's left with what's left of the star's nucleus. The outer layers of the star may have been destroyed by the explosion.
With no nuclear reactions to generate energy to counteract gravity, the mass of the core is so great that it undergoes a catastrophic collapse in which the pressure on the nucleus is so great that the electrons and protons are unable to overcome each other. The result of the core is a neutron star. They are small and pack in the entire mass of the dead star's core.
The most massive star ever found has a mass 2.35 times greater than our sun. The amount of material you could scoop from the surface of a neutron star would weigh as much as a mountain.
There are also sites of r-process nucleosynthesis when there is a merger of two or more neutron stars. The mass of Earth was 16,000 times greater in the form of r-process heavy elements, including ten Earth mass's worth of gold and Platinum, as a result of the merging of two star systems.
The 21st Century SETI has a verified account. We encourage you to follow us on social networking sites.
The U.S. Department of Energy has more information on the subject. The UK Science Technology Facilities Council uses neutrons in studies of Condensed Matter. The B2FH paper explains how elements can be created inside stars with the help of neutron capture.
Brian R. Martin is the author of Particle Physics.
Kaler wrote The Cambridge Encyclopedia of Stars.
The Collins Internet-Linked Dictionary of Physics is open.
In physics history this month. The American Physical Society has websites. The article can be found at: http://www.aps.org/publications/apsnews/200705/physics history.
A type of decay called neon decay. It's science direct. There is a new tab at the bottom of the page that says "neutron-decay".