A microorganism that can fix nitrogen and produce methane and ammonia has been enhanced by scientists at the Max Planck Institute for Marine Microbiology.
Life is dependent on carbon and nitrogen. One of the organisms that takes up key positions for the cycling of both of them is Meth anothermococcus thermolithotrophicus. The microbe is hidden behind the complicated name. M. thermolithotrophicus is a heat loving methanogen.
It lives in the ocean at temperatures of 65 C. Ammonia and methane can be used for biotechnological applications in agriculture.
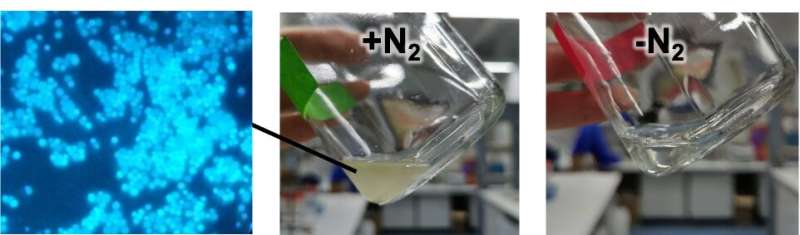
Two people from the Max Planck Institute for Marine Microbiology have succeeded in growing this microbe in a fermenter.
"It is very difficult to provide the perfect conditions for this microbe to thrive while fixing N 2 and keeping an eye on hydrogen and carbon dioxide levels," said Masla, who carried out the research as part of her PhD project. We were able to make them thrive in our lab and reach the highest cell densities reported so far.
Masla says that they collaborated with their colleagues to dig into the metabolism of M. thermolithotrophicus.
It was as if a bumblebee was a bumblebee.
The metabolism of M. thermolithotrophicus is a mystery because it originated on the early anoxic Earth. Methanogens don't get a lot of energy from methanogenesis compared to humans. Nitrogen needs huge amounts of energy to be fixed.
"They are a bit like bumblebees, which are theoretically too heavy to fly, but still, they do so," says senior author and leader of the Max Planck Research Group Microbial Metabolism. Despite the energy limitation, these fascinating microbes have been found to be the best nitrogen fixing organisms.
There is a nitrogenase.
Nitrogen is fixed by the nitrogenase that organisms use. Molybdenum is required for most nitrogenases. Molybdenum nitrogenase is a well-known symbiont in plants. They can be stopped by tungstate.
The scientists found that the growth of M. thermolithotrophicus was unaffected by tungstate. "Our microbe was dependent on Molybdenum to fix N 2 and not bothered by tungstate, which means an adaptation of metal-acquisition systems, making it even more robust for different potential applications," said Masla.
Ammonia production needs to be reconsidered.
Nitrogen is inserted into the biological cycle through Nitrogen fixation. The Haber-Bosch process is used to make ammonia with hydrogen under high temperatures and pressures. Most of the world's ammonia is produced using it.
The Haber-Bosch process consumes 2% of the world's energy output and releases up to 1% of global carbon emissions. People are looking for more sustainable ways to make ammonia.
The process used by M. thermolithotrophicus shows that there are still ways to increase the efficiency of ammonia production, and that they can be combined with the production of methane.
The study showed that the methanogen sacrificed its production of proteins to favor nitrogen capture, a smart strategy of energy reallocation. The next step will be to look into other parts of the metabolism, as well as the details of the process.
The research was published in the journal mbio.
Comparative Transcriptomics sheds light on the remodeling of Gene expression in the thermophilic methanogen meth anothermococcus thermolithotrophicus. There is a book titled "10128/mbio.02443-22".
Journal information: mBio