The Clinical Trials on Alzheimer's Disease meeting will be held in San Francisco at the end of November. The conference is where the latest progress in Alzheimer's research is made public for the first time.
The meeting is poised to be historic. Scientists expect to hear details of the first treatment that can change the course of Alzheimer's after more than a century of research. Nothing has been done to slow or reverse the decline of patients' brains. There is talk of a historic moment due to the fact that dementia and Alzheimer's are the No 1 killer in the UK and the seventh largest killer worldwide.
The optimism comes from a press statement from two companies. More than 2,000 people with early Alzheimer's disease were treated with lecanemab. The therapy slowed cognitive decline, the statement said, raising hopes that a drug might finally apply the brakes to Alzheimer's.
Researchers who have been unsuccessful in the search for Alzheimer's drugs were happy with the announcement. Even the most enthusiastic acknowledged that there were still questions. It was difficult to be certain that the claims stood up. The results of the trial will be presented at the San Francisco meeting on November 29th.

The debate started with Lecanemab. Many countries don't have enough money to pay for Antibody Drugs. It's not easy to give lecanemab, patients need to go to the clinic twice a month for an IV. Patients on the trial had regular scans for brain swelling and haemorrhages, a service many hospitals cannot offer.
Lecanemab might not be very effective. It is not known what the data will do to the burden inflicted by Alzheimer's. The benefits of the drug may not be noticed by patients. Some people think that the effect on Alzheimer's should be celebrated: it proves the disease can be defeated. It is a starting point to build on.
"Dementia is a global economic disaster as people are institutionalised while their disease progresses at huge cost to society and healthcare systems," says Prof Giovanna Mallucci, former centre director of the UK Dementia Research Institute at the University of Cambridge. You will begin to see an impact at the economic and medical level if you can slow the decline.

The situation is similar to the HIV crisis of the 1980's. The first anti-HIV drug was not perfect, but it paved the way for the current therapies. Prof Bart De Strooper is the director of the UK Dementia Research Institute at University College London. Now that we can find something, there is a lot of faith. We may be able to offer something decent to patients within the next few years.
The head of research at the Alzheimer's Society says that if it does slow cognitive decline, it means that we are modifying the disease. The real-world clinical benefit is something we need to understand, but it looks like the real deal to me. Everyone is saying that this is the beginning of disease-modifying treatments.
Alzheimer's is the leading cause of dementia around the world. The condition costs the UK £25 billion a year and is on course to double to £50 billion by the end of the century. As the disease progresses, people can find themselves lost in familiar places, have trouble with decisions, and experience mood swings. People die within eight years of being diagnosed with Alzheimer's disease.
If we think about Alzheimer’s in old age, I think most people have mixed forms of dementia
Alzheimer's is caused by the destruction of cells in the brain. On death, a patient's brain can weigh less than before, a reduction of more than 10%.
There is still debate about what causes the brain cells to die. Some families with early-onset Alzheimer's have been found to have abnormal clumps of a brainprotein called amyloid alpha. These plaques seem to help the formation of harmful tangles of another brainprotein called tau. The cells themselves accumulate these. The more tangles the worse the cognitive decline.
Inherited forms of Alzheimer's are not very common. The decline is likely to be driven by a messy mix of processes. Amyloid and tau are still in the picture, but other toxic proteins, chronic inflammation, cardiovascular problems, and faulty disposal of waste from the brain may all contribute. Most people with Alzheimer's don't have pure Alzheimer's according to De Strooper. They have different types of dementia. It's not always clear what drives the disease.
amyloid has been the focus of Alzheimer's drugs. Some aim to block the production of abnormal amyloid while others aim to remove it from the brain. There were more than a dozen final-stage trials of amyloid-targeting drugs between 2007 and 2019. Some made it worse.
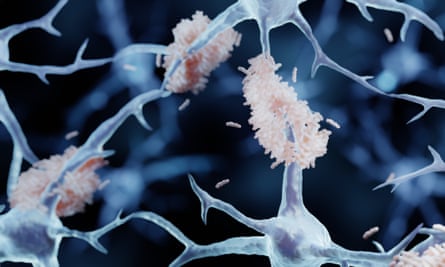
There were failures in the research community. The whole amyloid hypothesis was thrown out. Even if it was valid, amyloid wasn't the bestProtein to target Patients who already had Alzheimer's symptoms wereEnrolled in the trials. When the fire is raging, removing amyloid may not be enough. The early phase of the disease makes it difficult for people to notice. De Strooper says that the brain is made of plastic and can cope with a lot of damage.
The FDA gave the go-ahead to the first new drug for Alzheimer's in 20 years in June of last year. The decision to approve Aduhelm provoked a furore. The trial data failed to show a clear benefit.
The FDA granted accelerated approval because it could slow the progression of Alzheimer's if taken early enough. Several scientists resigned from the committee in protest of the ruling, which they said was the worst drug approval decision in US history.
The FDA will make a decision on lecanemab in January of next year. The effect of the lecanemab trial was small. The decline in cognitive function was less in those who took the drug. The people who were on lecanemab performed better than the people who were not on lecanemab. It may not mean a lot for individual patients.
The effect they have reported so far is very small. Prof Henderson says that it is not large enough to be clinically important. When the observed benefit is so marginal, there is a subjective element to the ratings. He is concerned that this could be a drug that has statistical significance without clinical significance.
One idea gaining ground in Alzheimer's research is that drugs need to be removed fast to have any hope of showing a benefit. The logic is laid out in a paper by two people. It will take time for the effects of amyloid removal to show up in thinking and memory tests, according to them. They suggest that if a drug doesn't push amyloid low enough, or takes years to do it, it won't help.
Mallucci sees positives in the results. If the results hold up, you can change the rate of decline of the disease. The individuals might not feel very different, but you can build on it.
Patients have been waiting too long for progress because of chronic under funding. De Strooper looked at the US medical database for dementia. He was able to find 255,000 studies. He searched for cancer and found almost five meters. He searched for Covid, a disease that didn't exist before the year 2019. More research has been done on Covid in the past three years than it has been in the past century.
It's striking that the comparison is with cancer. Cancer diagnosis and care have been improved by decades of funding. People should not wait more than 28 days from the time they are referred to be told if they have cancer. Two-thirds of patients with dementia are not diagnosed by the National Health Service. There is no mention of a time scale.
The full suite of diagnostic equipment is available for patients with cancer. A detailed diagnosis of the cancer is received by many people. Dementia diagnosis and care are behind the times. Alzheimer's patients will need an early diagnosis to benefit from amyloid-clearing treatments. Clinics in the UK are not close to being able to provide such services. When we have access to a drug that modifies disease but are unable to give it to those most likely to benefit, we could be in a situation in 25 years.
The hope may be completely different. To have a significant effect on the disease, a combination of drugs that hit different biological processes is needed. Mallucci believes that there will be a place for a small number of carefully selected patients. On a global scale, this isn't a feasible way to treat dementia. It is not feasible.
One idea is to give a vaccine that will prompt the patient's immune system to make a response to the problem. De Strooper believes that there are drugs that are worth looking at again. Mallucci believes that drugs that protect and revive brain cells are better for the aging brain. There are ways to boost the brain's ability to clear toxins. She has pioneered work on increasing the fitness of brain cells, as well as the ability to make new proteins, and on the "cold-shock" moleculeRBM3 which mammals release when they're cold. In mice, at least, these approaches help to protect against dementia by boosting memory and preventing brain cells from dying.
A second line of attack is to increase the amount of a compound called Bdnf, which could help cells by revitalizing them and building new connections. The University of California, San Diego is about to start a clinical trial that will test the effectiveness of a therapy for Alzheimer's.
The field requires more funding, more approaches, and more trials. He says that dementia can be made a chronic condition, one you live with and die with, but don't die from. We will see it in dementia because we have seen it work in other major conditions. The amyloid-clearing drugs are only the beginning.