New avenues of exploration in transitional metal catalysis are provided by theory-guided development of an easier, more versatile process.
The Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) has discovered a key to the creation of a tool that could greatly expand the variety of reactions possible with metals. The team has taken a well-established set of compounds that can be used to make transition metal catalysts and developed a simple, radical-based reaction for creating unsymmetric versions of them. New possibilities for designing transition metal catalysts are opened up by easier access to a wider variety of unsymmetrical compounds.
This research focuses on a class of compounds called 1,2-bis(diphenylphosphino)ethane derivatives. DPPEs are attached to the metal center of a catalyst in two different places. Structural variety and reactivity are limited by the fact that each attachment arm is the same. The versatile method for developing unsymmetric DPPEs using ethylene was reported in the study.
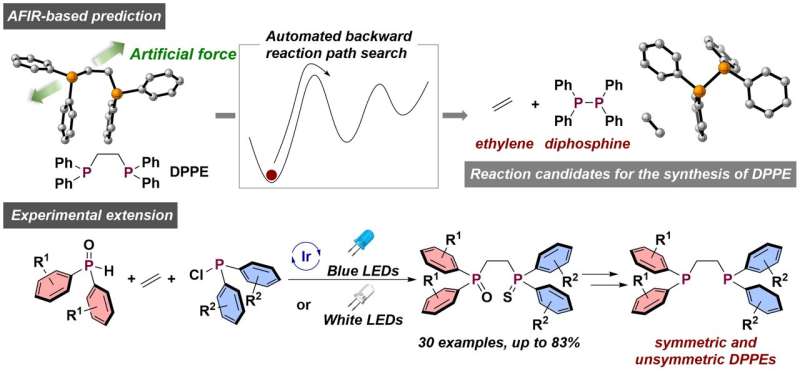
Researchers used quantum chemical computations to find possible starting materials that could react to DPPE. The process of phosphine radicals reacting with ethylene was shown to be viable in computations.
The team verified a simple process for making symmetric DPPEs by mixing three readily available compounds. Previous methods involved multiple steps and used highly reactive compounds.
A wide range of electronic properties and sizes were covered by the unsymmetric DPPEs that were mixed with both chlorophosphines and phosphine oxides. There is a push-pull effect when the size and electronic properties are different. The use of a photocatalyst gave the best yield.
The team used one of the unsymmetric DPPE derivatives to form metal complexes. They compared the properties of the two derivatives. The potential for unsymmetrical DPPE derivatives to enable different reactivity when used as catalysts was shown by the two complexes. The use of a low-cost abundant material is an advantage of this method.
Hideaki Takano, the lead author, said that they succeeded in making DPPE derivatives that are useful as catalysts. The result was realized due to the synergistic effect of using AFIR quantum chemical computations. I would like to use the method we reported here to develop new reactions.
Hideaki Takano et al, A theory-driven synthesis of symmetric and unsymmetric 1,2-bis(diphenylphosphino)ethane analogues, was published in Nature Communications.
Journal information: Nature Communications