The National University of Singapore developed a method to synthesise bi- and tri-flavones.
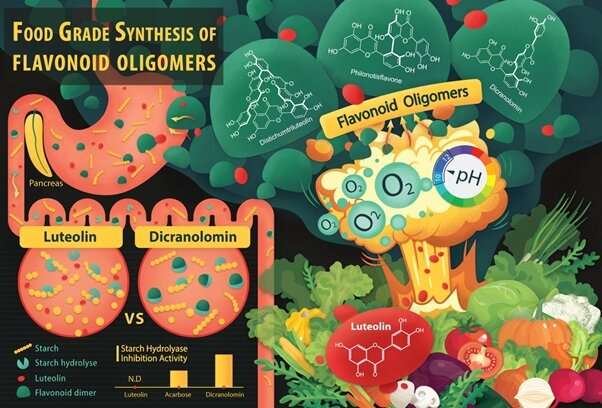
Plants with a wide range of health promoting benefits are the source of fonoids. Reducing the risk of non-communicable diseases such as hypertension, diabetes and chronic inflammation is part of this. It is impractical to get the more potent flavonoid dimers and oligomers from natural sources because they are only present in trace amounts. It is difficult to make these molecule due to their complex structures.
A professor from the Department of Food Science and Technology at the National University of Singapore and his team developed a catalyst-free method to drive thecoupling reaction between flavone monomers. In the process of generating a high yield, the hydrogen acceptor in alkaline water acts as a hydrogen atom.
The researchers used this method to make more than 40 flavone dimers and trimers. The work is being done by Professor Houk from the University of California.
The reaction was developed from a discovery by a PhD student. Big discoveries can be made by paying attention to details.
This finding opens up an efficient and eco-friendly way to synthesise flavone dimers and oligomers, which could be used to fight disease and improve human health. They can be used as active ingredients or pharmaceutical agents to treat diseases.
With the small library of bi- and tri-flavones synthesized from their newly discovered method, Prof Huang and his research team have been screening the bioactivity of these flavonoids and studying the relationship between them. They are trying to expand the scope of the synthetic reaction to make oligomers.
Interested partners are welcome to study the bioactivities of the bi- and tri-FLavones. The team believes that the library will help promote human health and prevent diseases.
In Nature Communications, there is more information about the Oxygen-mediated oxidation of flavones.
Journal information: Nature Communications