Fossil fuels have been the primary source of concentrated energy for the past two centuries. Fossil fuel use has a negative impact on the climate.
Nature has no solution for the amount of energy we use, according to a University of Chicago chemist. He said that we will have to do better than nature.
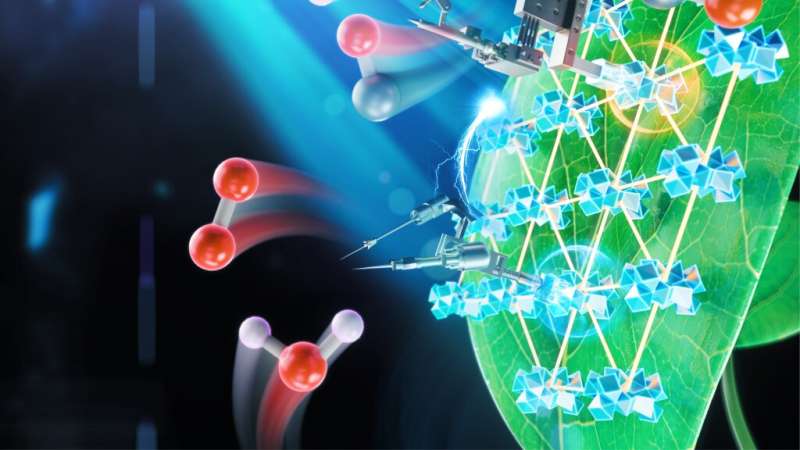
Artificial photosynthesis is a possibility that scientists are exploring. The chemical equipment in a single leaf is difficult to use.
An innovative new system for artificial photosynthesis that is more productive than previous artificial systems was shown in a Nature Catalysis study. Artificial photosynthesis, which uses carbon dioxide and water instead of carbon dioxide and water, can produce fuel.
Though it has a long way to go before it becomes a way for you to fuel your car every day, the method gives scientists a new direction to explore, and may be useful in the short term for production of other chemicals.
"This is a huge improvement on existing systems, but just as importantly, we were able to lay out a very clear understanding of how this artificial system works at the molecule level, which has not been accomplished before," said Lin.
Something else will be needed.
We wouldn't be here without natural photosynthesis. Lin said that it made the food we eat. It won't be efficient enough to supply fuel for us to drive cars, so we'll need something else.
The problem is that our cars don't need much more concentrated energy than we do, and that's a problem. Researchers looking to create alternates to fossil fuels need to re-engineer the process to create more energy dense fuels.
In nature, the process of photosynthesis is performed by many different parts of the body. A long string of hydrogen-oxygen-carbon compounds are made when they take in water and carbon dioxide, break the molecule apart, and rearrange the atoms. Scientists need to change the reactions to make a different arrangement with just hydrogen around the carbon core.
People have been tinkering with it for decades, trying to get closer to the efficiency of nature.
Lin and his lab team thought that they could add something that hasn't been included in artificial photosynthesis systems yet.
A metal-organic framework is a class of compounds made up of metal ion and organic linking molecule. In order to give the maximum surface area for chemical reactions, they designed the MOFs as a single layer and submerged everything in a solution that included a cobalt compound. They tried to find out which worked best with the new additions.
The process that breaks apart water and the process that adds electrons and protons to carbon dioxide were both improved. The reaction went more efficiently with the help of the Amino Acids.
Artificial photosynthesis has a long way to go before it can be useful for widespread use. Lin said that it would take many orders of magnitude to make enough methane for consumption.
You need a lot of fuel for the breakthrough to have an impact, but smaller quantities of some molecule, such as the starting materials to make pharmaceutical drugs and nylons, could be very useful.
Many of the fundamental processes are the same. Good chemistries can be plugged into a lot of systems.
There are biomimetic active sites on monolayer metal and organic frameworks for artificial photosynthesis.
Journal information: Nature Catalysis