It's like she's watching an attempted break-in on a home security camera when she pulls up the video from her PhD.
The person looking for a point of entry doesn't need to set a foot inside. This is a different type of person. It is a Viruses.
Filmed over two and a half minutes, the footage shows a tiny virus particle, thousands of times smaller than a grain of sand, as it lurches and bobs among tightly packed humans.
The virus skims along the surface of a cell, but doesn't stick, for a short time. If this were an actual home break-in, Johnson says, this would be the part where the person hasn't broken the window.
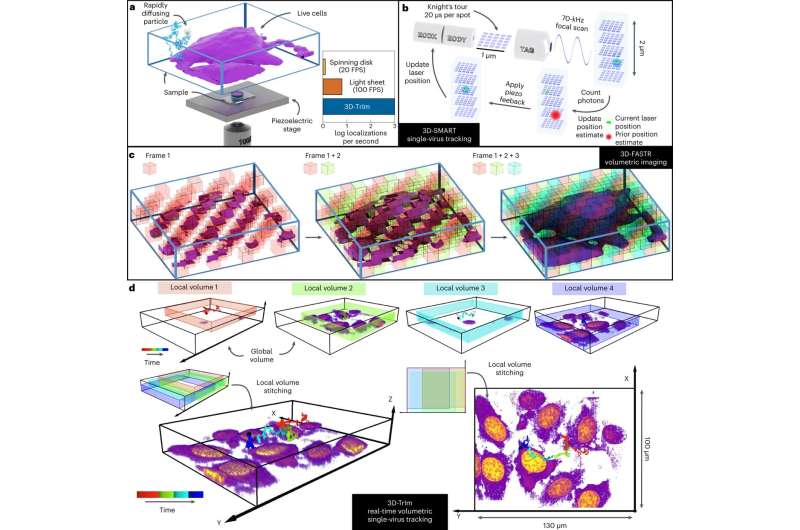
Kevin Welsher is a chemistry professor at Duke University. Welsher and Jack Exell have collaborated to come up with a way to capture real-time 3D footage of viruses as they approach their cellular targets. The journal Nature Methods contains their research.
Every day we take in millions of Viruses. Some of them can be dangerous, such as the viruses that cause the flu or COVID-19.
When a Viruses enters a cell it hijacks the cellular machinery to make copies of itself A virus has to get to the cell first before it can break in.
One of the first lines of defense against infections is the mucus and cells in the airway and gut.
The researchers wanted to understand how viruses are able to penetrate these defenses. How do viruses get around? Welsher spoke. It has long been difficult to watch the critical early moments before infections begin.
Viruses move two to three orders of magnitude quicker in the unConfined space outside of the cell than they do inside. Viruses are hundreds of times smaller than cells and this makes them difficult to image.
This is a difficult problem to study because of that. It's like taking a picture of a person in front of a skyscraper. It's not possible to see the details of the person in front of the skyscraper with a single picture.
The team developed a new method that combines two microscopes in one. The first microscope "locks on" to the fast- moving virus, using a laser to calculate and update its position tens of thousands of times per second. The microscope stage constantly adjusts to keep it in focus as the viruses bounce around in the cell.
3D images of the surrounding cells are taken by the second microscope. Welsher said that the combined effect is similar to navigating with Google Maps, showing your current location as you drive, as well as the terrain, landmarks and the overall lay of the land.
When presenting his work, Johnson sometimes gets asked if it's a video game or a simulation. This is from a real microscope.
The researchers can't just watch a healthy person breathe in virus particles from a sick person. They need to attach a fluorescent label to a virus before they can use a microscope to see it. They can only keep an eye on a virus for a short time.
Exell said that the biggest challenge for them is to make brighter viruses.
Welsher hopes the technique will make it possible to follow infections in action beyond the coverslip and in more realistic environments.
Welsher said that this is the real promise of the method. We think we have the chance to do that now.
There is more information about Capturing the start point of the virus–cell interaction with high-speed 3D single-viruses. It's 10.1038
Journal information: Nature Methods
Citation: Watch a virus in the moments right before it attacks (2022, November 11) retrieved 11 November 2022 from https://phys.org/news/2022-11-virus-moments.html This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.