To grow and spread around the human body, cancer cells need copper ion binding. Potential new drug targets in the fight against cancer have been opened up by new research about how cancer-related proteins bind the metal.
The metal copper is needed by human cells to carry out important biological processes. Studies have shown that cancer patients have higher levels of copper in their blood than healthy patients. The more active the copperbinding proteins, the higher the level of copper.
Pernilla Wittung-Stafshede is a professor of chemical biology at the University of Technology, Sweden.
The majority of cancer-related deaths are due to the fact that secondary tumors form in different parts of the body. Cancer cells use a signaling system called Memo1 to grow and spread. The ability to form metastases decreases when the gene for Memo1 is inactivated.
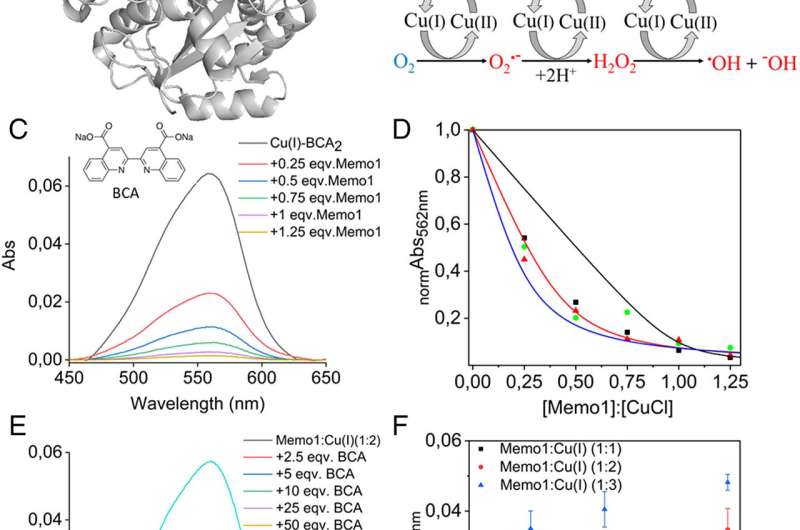
There is a connection between Memo1 and copper. In a new study published in the journal PNAS, the researchers looked at the ability of Memo1 to bind copper ion through a series of test tube experiments.
The reduced form of copper was found to be binding with theprotein. Most living cells have this form of copper ion. Reduction of copper in the body contributes to the damage that happens to the cells. When Memo1 interacted with copper, the metal's toxic reactions were blocked.
A lot of copper can cause chemical reactions that are harmful to the cancer cells. Pernilla Wittung-Stafshede is one of the study's lead authors.
Memo1 can form a complex with Atox1 in our cells. The research team has shown that copper helps breast cancer cells to move and form metastases. The findings of the new study show that copper and copper-binding proteins could be targets in the future.
When we looked at breast cancer cells, we found that Memo1 and Atox1 were close to each other. According to Pernilla Wittung-Stafshede, the exchange of copper between these proteins can take place in cancer cells as well as in test tubes.
The researchers want to know how the presence of copper affects Memo1's activity in cancer development.
Pernilla Wittung-Stafshede says that when we expand our knowledge of the role of copper-binding proteins in cancer cells, we also open the door to new treatments.
Xiaolu Zhang et al., Memo1 bind reduced copper ion, interacts with copper chaperone Atox1, and protects against copper-mediated redox activity in the body. 10.1073/pnas.2206905
Journal information: Proceedings of the National Academy of Sciences