A thin layer of water forms on the surface of ice even though it is well below zero degrees. The ice has melted even though the temperature is below the melting point. snowballs stick together because of the liquid layer on ice.
It wasn't until about 140 years later that it was scientifically confirmed. A liquid layer only a few nanometers thick forms on the surface of the otherwise solid material when surface melting occurs.
Surface melting is important in view of technical applications because it plays a crucial role in their use.
The effect of taking an ice cube out of the freezer and exposing it to ambient temperature is not related to this process. The surface of an ice cube is warmer than the ice cube's interior, which causes it to melt on its surface.
Glass has a surface melting problem.
The thin liquid layer on the surface is usually detected by scattering experiments, which are very sensitive to atomic order. Since liquids are not arranged in a regular pattern, such techniques can resolve the appearance of a thin liquid film on top of the solid
The approach doesn't work for glasses because there is no difference in the atomic order between the solid and liquid. Experiments haven't explored surface melting of glasses.
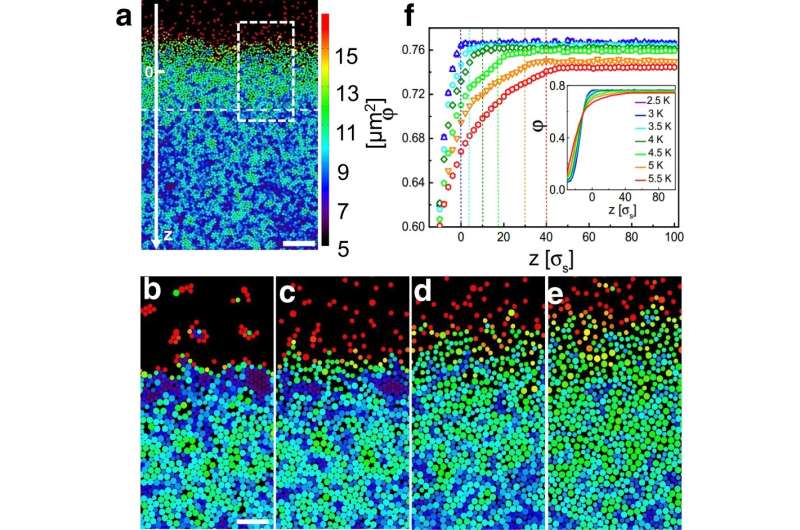
The physicists at the University of Konstanz used a trick called colloids to overcome the difficulties. These particles are 10,000 times larger and can be seen under a microscope.
The researchers were able to demonstrate the process of surface melting in a glass with particles near the surface. Since the particle density at the surface is lower than in the underlying bulk material, it's not surprising that such behavior would occur. Particles close to the surface have more space to move quickly.
It was a surprising discovery.
The particle mobility is still higher than the bulk material even if the density reaches the bulk value.
This previously unknown layer is up to 30 particle diameter thick and continues from the surface into the deeper regions of the solid in a streak-like pattern. It combines liquid and solid features and has interesting material properties.
Thin films have different properties depending on their thickness. This property is being used to make thin ionic conductors in batteries, which are found to have a higher ionic conductivity than thick films. The new insights gained from the experiments will make this behavior better for technical applications.
The research was published in a peer-reviewed journal.
There is more information about the surface melting of a glass.