There are a lot of drugs that target the membranes. They are in the middle of our cells. The substances are moved in and out of the environment. It's difficult to store and extract them for observation.
An innovative method to study their structure in their native environment has been developed by a team from the University of Lausanne. The technique is based on magnetism. Future development of new drugs could be aided by these results.
Each cell in a living creature has a cell membrane. There is a double layer oflipids. The cell's contents are separated from its environment to regulate the substances that can enter or leave the cell. There are two types of cells, the one attached to this one and the one attached to another.
They are located at the interface between the outside and inside of the cell and are involved in cell signaling. More than half of the current drug targets are found in the form of MEA.
There are difficult objects to look at.
The study of their structure is important. To understand them, scientists need to separate them from all other proteins. It's not possible to study the proteins in a solution. Liquid solutions made of detergents are used to maintain them. They can be inserted into a variety of different types of membranes.
These strategies remove them from their environment and don't allow them to be observed in situ. Drug development might be misled by the different structural properties of proteins outside of their environment.
A new way of doing things.
A team led by Enrica Bordignon, full professor in the Department of Physical Chemistry at the UNIGE Faculty of Science, has developed a new method for studying the action of a group of genes. The research team used a specific tool to accomplish this.
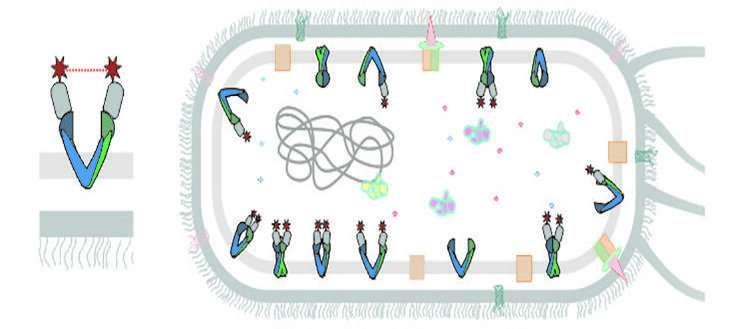
Enrica Bordignon explained that these are fragments of antibodies that are able to recognize and bind to a specific target. The scientists have been able to directly report on the structure of the transporter with the help of the artificially produced conjugates.
"Inserted into E. coli cells, two nanobodies target the desired MembraneProtein on the inside of the cell and attach to it," says Markus A. Scientists from the University of Osnabrueck, Germany, as well as the University ofSouthampton, U.K., were part of the multi-disciplinary team.
There are new drug targets.
A magnetic probe was attached to each body beforehand. The distance between the two magnetic probes in cells can be measured using our EPR methods. The technique is called EPR. One millionth of a millimeter is how far the distance is measured. Enrica Bordignon says, "For the first time, we have been able to get a clear picture of the structure of a MembraneProtein in its real environment and we could follow the change in it."
The development of this new strategy was the result of excellent and challenging teamwork between our two groups. The project was a success because of the resilience of the two authors.
The new strategy allows a precise determination of the properties of the membranes. It's possible to understand how certain substances get into and out of the cell. The method has the advantage of being easy to use. It could be used to better understand the phenomenon of multi-drug resistance, which is caused by the rejection of anti- cancer drugs outside the cell.
More information: Laura Galazzo et al, The ABC transporter MsbA adopts the wide inward-open conformation in E. coli cells, Science Advances (2022). DOI: 10.1126/sciadv.abn6845 Journal information: Science Advances