The phrase five states of matter is outdated as there are many more states of matter. Four of these occur naturally, while others are only fleeting in the lab.
All matter is composed of atoms, which are made up of protons, neutrons and electrons.
Washington State University says that atoms are the building blocks for all kinds of matter. The U.S. Energy Information Administration states that both atoms and molecule are held together by a form of potential energy.
The number of atoms in the observable universe is related.
There are four states of matter. The Bose-Einstein condensates are only made in the lab. Fermionic condensates and time crystals can be made in a lab under extreme conditions. There is a chain-melted state that is both a solid and liquid at the same time.
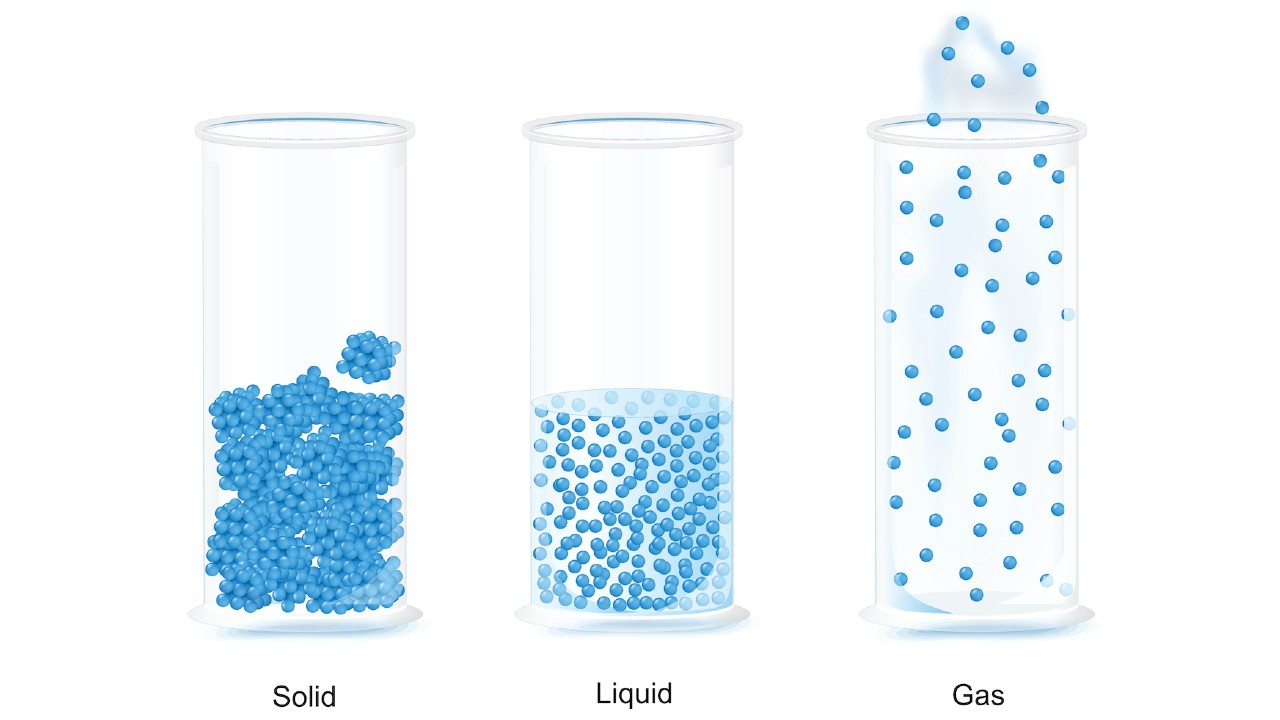
Particles are packed tightly together in a solid. The atoms have a small vibrate but they are fixed in their positions. Particles in a solid have very low energy levels.
Solids don't fit in with the shape of the container in which they are placed. Solids have a high density and are tightly packed.
The particles in a liquid are more tightly packed than in a solid and are able to flow around each other. The liquid will fit in the container it's in.
Liquids are much harder to compress than solid ones.
The particles in a gas have a lot of space between them. There is no definite shape or volume of the gas. If unConfined, the particles of a gas will spread out and the gas will expand to fill the container. The space between particles is reduced when a gas is put under pressure by reducing the volume of the container.
According to the Jefferson Laboratory, the most common state of matter in the universe isplasma. The stars like the sun are very hot.
Particles with high energy are called plasm. The noble gases can be used to make glowing signs by ionizing them.
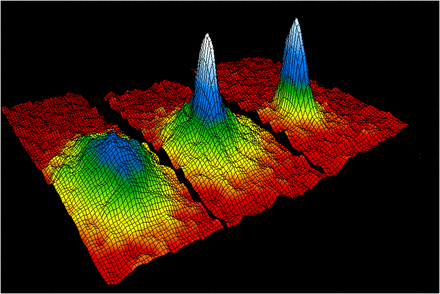
Scientists created a bec in 1995. The rubidium was cooled to a few degrees of absolute zero using a combination of lasers and magnets. The motion of the molecule is very close to stopping. The atoms clump together because there is almost no energy transfer from one atom to another. Just one "super atom" is all there is.
BECs are used to understand quantum mechanics. As light passes through a BEC, it seems to slow down. Many of the properties of a superfluid are found in a BEC. Black holes might have conditions that are similar to BECs.
There are many states of matter that have been created. During the transformation between the state of liquid and solid, glass becomes a new state of matter referred to as liquid glass.
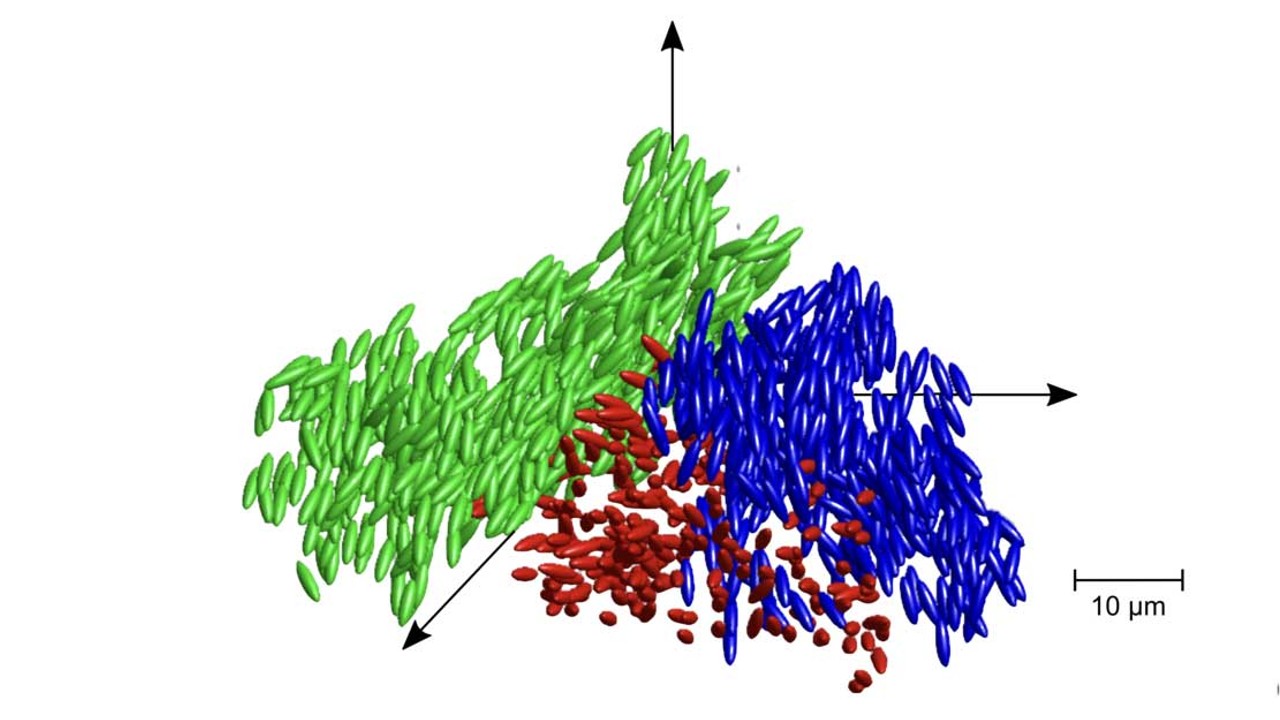
Liquid glass is a mixture of particles that are larger than a single atom. Unlike glass, when a substance transforms from a liquid to a solid, the molecules are arranged in a structure that iscrystallised. Liquid glass is more flexible than solid glass, but can't rotation.
The kind of evidence for the interplay between critical fluctuations and glassy arrest that the scientific community has been after for quite some time was provided by the senior author of the study.
How do you determine the size of an atom?

Frank Wilczek proposed the idea of time crystals in 2012 and they have the ability to cycle between states of energy. They are able to avoid the second law of thermodynamics because they don't reach equilibrium or a steady state.
A time crystal was created in a lab and made in a quantum computer, which lasted for 100 seconds before falling apart.
The fermionic condensates are a sister phase to the BEC. Superfluids are those that can flow with no resistance. They are made up of fermions and have odd atomic numbers. Scientists have to get perturbons to pair up to make this phase.
Scientists make the matter very cold in order to do this. The scientists at JILA in Boulder, Colorado cooled a cloud of half a million atoms to less than a millionth of a degree above absolute. The superconductivity that occurs in electron pairs is caused by this.
Matter moves from one state to another when energy is added or removed. Adding heat to liquid water causes it to become steam. Liquid water becomes ice due to the removal of energy from it. Motion and pressure can cause physical changes according to the Abridged Science for High School Students.
Warm and cold.
When heat is applied to a solid it causes its particles to move further apart. The solid will start to melt when it reaches a certain temperature and pressure.

The temperature of the substance will not increase until the entire sample reaches the same physical state as the two states of matter. If you put ice into a glass of water and leave it out, it will come to the same temperature. The ice cube will stay at 32 degrees Fahrenheit (0 degrees Celsius) until it starts melting from the water.
When heat is removed from a liquid, the particles begin to settle in one place. The liquid becomes a solid when it reaches a certain temperature.
The preliminary examination.
Sublimation is the process of converting a solid into a gas without going through liquid phases. Dry ice and frozen carbon dioxide are some of the volatile substances that will undergo submerging.

It's called Vaporization.
Vaporization is the process of converting a liquid to a gas.
The particles of a liquid often collide with each other. When enough energy is transferred to particles near the surface they may be knocked away from the sample. The energy transferred to surface molecule causes them to escape and the liquid cools as they evaporate.
Vapor bubbles form below the surface when liquid is boiled. The boiling point is the temperature and pressure of a liquid.
Deposition and condensation.
According to the U.S. Geological Survey, condensation happens when a gas loses energy. Water condenses into liquid water at a certain point in time.
When a gas transforms into a solid, it's called deposition. When the air touches a blade of grass it becomes cooler than the rest of the air.
The updated article was published in October of 2022.