The National Institute for Physiological Sciences is a national institute.
The process of pumping sodium ion was visualized directly by a team of specialists in the field. They should help develop a better understanding of cellular energy-conversion motor mechanisms.
The paper about their investigation was published in the National Academy of Sciences.
Many will remember from secondary-school biology how in complex cells, the powerhouse of the cell, is a small molecule called ATP synthase, which can be found in the cell's mitochondria. A hydroelectric plant takes advantage of the rush of water behind a dam to turn a turbine that drives a generator.
In the same way, the "rotors" of an ATP synthase protein use a rush of protons from a region on one side of the membranes with a high concentration to a region on the other side of low concentration.
The vacuolar ATPase that uses the energy from the ATP to produce a protons is called the V-ATPase. In a similar way to how some hydroelectric plants that operate like large batteries can work in reverse, using electricity to pump water back up into a lake.
Studies have been done on model types of V-ATPases to understand how the two motor proteins are able to work together.
Only a small number of studies have been done on other types of V-ATPases that may have different functions. It was not easy to understand the mechanism of converting one type of energy to another.
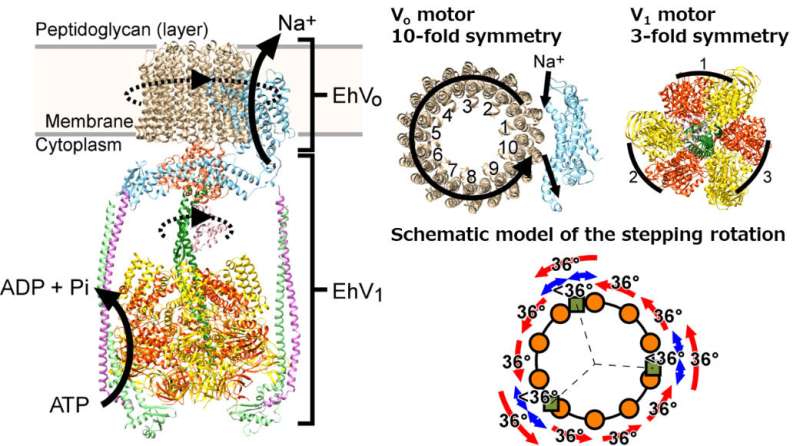
V-ATPases are capable of pumping both protons and Na +. In the bacterium Enterococcus hirae, it is possible to maintain desired Na + pump levels inside the cell.
There is a unit of the energy currency that is hydrolyzed and used to drive the V 1 motor. It does this in a way that's smart. EhV 1 rotates at a rate of 120 per "expenditure" of ATP.
The scientists found that each 120 step of the EhV 1 is divided into sub-steps of 40 and 80. The scientists were not sure how steps and pauses of EhV o happened.
There was a discrepancy between the two motor. The EhV o motor is translated from the EhV 1 motor. The latter motor has a ring of ten subunits which rotates in the membranes. The rotation of this c 10 -ring transports the sodium ion.
There is a mismatch between the number of sodium ion transported and the number of ATP spent per rotation.
Professor Iino said that each full turn costs threeATP or oneATP per 120 step of the EhV1 motor. 3.3 is one and a third of the total sodium ion count. It's not possible to have a third of asodium ion. What is happening?
The scientists knew what they were talking about when they said the rotation of the motor waselastic. The symmetry mismatch in these organisms can be alleviated by large changes in the peripheral stalks of the proteins. Due to the multiple peripheral stalks of the E. hirae V-ATPase, the mismatch could be fixed through additional pauses during rotation.
The only way to find out which of the two options was happening was to see the steps and pauses in the E. hirae V-ATPase motor's movement.
Using a 40 nanometer gold probe that could be tracked with a high-speed camera, the researchers found that the binding of sodium ion and binding ofATP to different parts of theProtein occur at different angles. The rigid component requires 13 pauses to solve the mismatch and a third of a sodium ion.
The results show that the multiple peripheral stalks of the V-ATPases are more rigidly coupled than the single peripheral stalks of theATPases.
The way for a comprehensive understanding of the mechanisms involved with ATPases is expected to be paved by the team's image of the V-ATPase motor.
More information: Akihiro Otomo et al, Direct observation of stepping rotation of V-ATPase reveals rigid component in coupling between V o and V 1 motors, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2210204119 Journal information: Proceedings of the National Academy of Sciences Provided by National Institute for Physiological Sciences