A new approach to analyzing carbon will lead to the discovery of new materials.
We use, eat, and create materials. We sometimes invent them by accident. Making useful materials is a long and expensive process.
An automated process for identifying and exploring promising new materials has been demonstrated by scientists at the U.S. Department of Energy's (DOE) Argonne National Laboratory. The approach could speed up the discovery of useful materials.
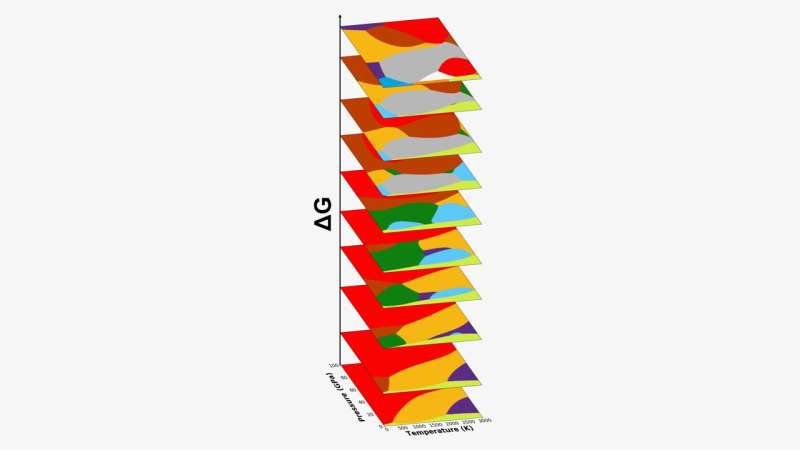
The single carbon element was used as a prototype to predict the way in which atoms order themselves under different temperatures and pressures. Phase diagrams are maps that help scientists find new and useful states of matter.
Pierre Darancet said that they trained a computer to probe, question and learn how carbon atoms could be organized to create phases that we might not find on earth or that we don't fully understand. The more this process is handled by a computer, the more materials science we can do.
It's equilibrium and beyond.
The atomic structure of a material can change. New ways to arrange atoms are being explored by scientists. Changing the atomic structure of a material can be done by changing the pressure and temperature.
Structural changes are common in water. Water is most stable at room temperature. Solid ice will form if the temperature is decreased enough.
The materials are both made of carbon atoms, but they are arranged differently. Graphene is a more stable form of carbon than diamonds. Under extreme pressure and heat, it is possible to make diamonds. Scientists call the diamond a metastable state when it's removed from extreme conditions.
Hundreds of these metastable states of carbon were mapped by the ML algorithm.
It's difficult to predict and produce states of matter that aren't near equilibrium conditions, according to an experimentalist. We can explore the little-known regions on the maps that aren't otherwise accessible or that we don't know about.
Ensuring the integrity of the program.
Synthetic data is produced through simulation and approximates the results scientists would get in an experiment. Computational chemistry tools were used to generate the dataset.
The training data was produced using a high performance computing cluster at a DOE office of science. The National Energy Research Scientific Computing Center was one of the DOE user facilities.
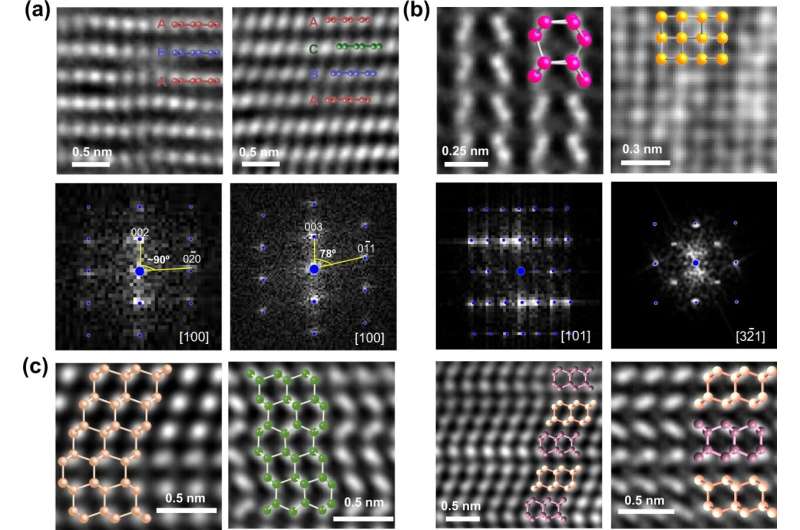
The team verified its efficacy by using actual samples and a transmission electron microscope.
The computer-generated phase diagrams affirmed and shed light on several unexplained experiments.
The n-diamond is a state of carbon that has puzzled scientists since it was discovered over 30 years ago. New and surprising predictions about structural features of n-diamond were made by the algorithm, and it proved to hold up even with high-profile phases.
Several phases that have not yet been reported in the scientific literature have been synthesised by the team. The structures of the samples match predictions.
A lead author on the study said that it can take several experimental trials and years of effort to synthesise materials with exotic properties. The time for the realization of exotic materials could be reduced by the use of machine learning.
The study only looked at carbon. The scientists want to apply the same approach to systems of more than one element. The discovery and design of useful materials could be impacted by applying machine learning to more complex systems.
The study was published in Nature Communications.
More information: Srilok Srinivasan et al, Machine learning the metastable phase diagram of covalently bonded carbon, Nature Communications (2022). DOI: 10.1038/s41467-022-30820-8 Journal information: Nature Communications