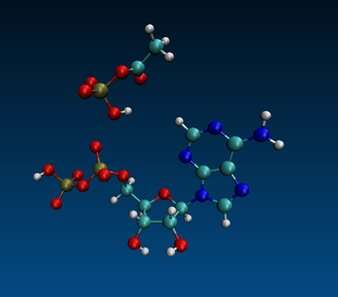
A simple two-carbon compound may have been a crucial player in the evolution of metabolism before cells.
All cells use adenosine triphosphate as anenergy intermediate. Most types of cellular functions are powered by the release of energy from the addition of aphosphate toADP. It takes six separate steps to build a complex chemical structure from scratch, and it's possible that some other compound may have played a part.
Lane and colleagues believed that the two-carbon compound acetylphosphate was the most likely candidate. A host of questions remained after the demonstration of AcP in water, including whether other small molecules might work as well, whether AcP is specific forADP, or whether it could function just as well with diphosphates.
The authors looked at all these questions. Drawing on data and hypotheses about the chemical conditions of the Earth before life arose, they tested the ability of other ion and minerals to do the same thing. They tested a panel of other small organicmolecules for their ability to phosphorylate ADP, none were as effective as AcP, and only one had any significant activity at all. The other nucleoside diphosphates didn't accept aphosphate from AcP.
Over time, with the emergence of suitable catalysts, ATP could eventually replace AcP as a ubiquitous phosphate donor, and promote the creation of RNA, DNA and proteins.
Silvana Pinna thinks that it might be possible to formATP from ADP under certain conditions. Several phosphorylating agents and metal ion catalysts were thought to work. It was surprising to discover that the reaction is very specific, and life still uses some of the same molecules. It's important that this happens in water under mild, life-compatible conditions.
More information: A prebiotic basis for ATP as the universal energy currency. PLoS Biology (2022). DOI: 10.1371/journal.pbio.3001437 Journal information: PLoS Biology