A promising cancer-fighting compound can be produced in the lab using a rapid and sustainable method. The only natural source of the compound is a single plant that grows in a small rainforest region of Northeastern Australia.
tigilanol tiglate is a compound that promotes a local immune response against tumors. The response breaks the tumors blood vessels and kills it. EBC-46 has a high success rate in treating cancer in dogs.
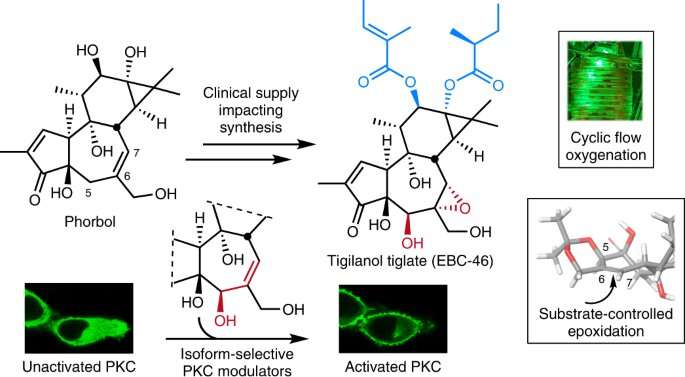
The complex structure of EBC-46 made it difficult to make it in a laboratory. Thanks to a clever process, the researchers were able to demonstrate for the first time how to transform abundant, plant-based starting material into EBC 46.
This process can produce EBC-46 "analogs" that are similar to each other but which could prove even more effective and potentially treat a wide range of other serious maladies. AIDS, multiplesclerosis, and Alzheimer's disease share the same biological pathways that are impacted by EBC-46's target.
Paul Wender is the Francis W. Bergstrom Professor in the School of Humanities and Sciences and the corresponding author of a study about the synthesis of EBC 46. It's great to be able to make EBC-46 in the lab.
The five co-authors of the study are all PhD students in Wender's lab.
The research team was very happy with the EBC-46 breakthrough. "If you were to visit the lab the first few weeks after they succeeded, you would see my stellar colleagues smiling from ear to ear," said Wender. They were able to do something that a lot of people thought was impossible.

From a far away place.
An automated drug candidate screening process by Q Biotics turned up Tigilanol tiglate. The compound is found in the seeds of the blushwood tree. When eaten, the tigilanol tiglate-rich seeds can cause nausea and vomiting.
Small amounts of EBC-46 can be injected directly into some tumors. EBC-46 is proposed to cause certain forms of PKC to be activated in order to cause an immune response to the cancer cells. The inflammation causes the tumor's vasculature to leak and cause the growth to die. There are ways of delivering EBC 46 to internal tumors that are being investigated.
Mast cell cancer, the most common skin tumors in dogs, can be treated with an EBC-46–based medication, which was approved by both the European Medicines Agency and the Food and Drug Administration. The cure rate after a single injection was 75% and after a second injection was 85%. There are currently clinical trials for skin, head and neck, and soft tissue cancers.
Scientists are considering setting up special plantations for blushwood trees because of the emerging research and clinical needs. There are a number of issues that need to be addressed. The right type of pollinating animals must be on hand, as well as trees that are planted in appropriate densities and distances to aid pollination. The trees are affected by seasonal and climate changes. Land use problems can be caused by setting aside plots for trees.
"For sustainable, reliable production of EBC 46 in the quantities we need, we really need to go the synthetic route."
EBC 46 is being made from scratch.
The plant-derived compound phorbol is a good starting point for EBC 46. There are more than 7,000 plant species that produce phor Bol derivatives and phor Bol-rich seeds. The herb used in traditional Chinese medicine is called purge croton.
The preparation of EBC 46 is similar to an everyday experience. Wender said that making coffee in the morning is similar to buying a sack of seeds. You grind up the seeds and use a hot solvent to get the active ingredient.
The researchers had to figure out how to bedeck the B ring with oxygen atoms after processing the oil. This is needed to allow EBC-46 to interact with PKC.
The researchers relied on instruments at the Neuroscience Microscopy Service, the Cancer Institute Proteomics/Mass Spectrometry Shared Resource, and the Sherlock cluster for computer modeling.
The team was able to add extra oxygen atoms to the B ring by conducting a so-called ene reaction under flow conditions. The team was able to get the desired spatial arrangements of the atoms by introducing other B ring groups. Only four to six steps were needed to get to the EBC-46 itself.
Wender hopes that this breakthrough approach will speed up research into potentially revolutionary new treatments.
"As we learn more about how cells function, we're learning more about how we can control that function." Control of function is important in dealing with cells that go rogue in diseases.
Wender is a fellow of Sarafan ChEM-H and a member of several other organizations.
More information: Paul A. Wender et al, Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues, Nature Chemistry (2022). DOI: 10.1038/s41557-022-01048-2 Journal information: Nature Chemistry