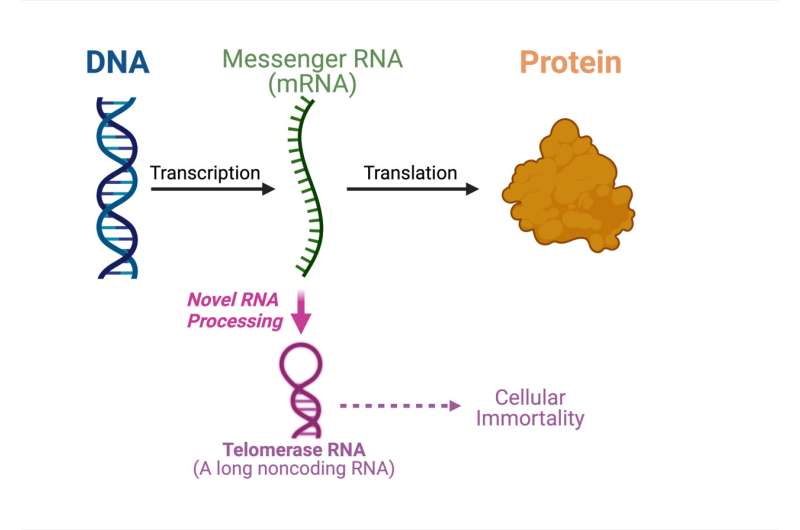
For the first time, a study led by Julian Chen and his group in Arizona State University's School of Molecular Sciences and the Biodesign Institute's Center for the Mechanism of Evolution has found an unprecedented pathway that produces telomerase.
The order in which genetic information is transferred from one cell to another is specified in the central dogma. The genes from the nucleus of the cell to the cytoplasm are carried by messenger RNA. The messenger acts as a building block.
There are many ribonucleic acids that aren't used to make proteins. The majority of the human genome is used to make noncodingRNAs that don't code for a specific sequence of genes.
One of the noncodingRNAs that assembles with telomerase is called telomerase. Cell immortality is dependent on telomerase. In this study, Chen's group shows that a telomerase is processed from a transcript of a messenger RNA.
The paper's findings are paradigm- shifting. There is a dual function mRNA that can be used to make a noncoding telomerase and it is unique. We need to do a lot more research to understand the underlying mechanism.
Important medical applications have arisen from basic research on the metabolism. Several COVID-19 vaccines use messengerRNA as a means of production. Our bodies absorb the vaccine's mRNA molecule after it is degraded.
The risks of being deleteriously and permanently incorporated into our DNA are mitigated by this new approach. This work may lead to innovative ways of making future vaccine.
In this study, Chen's group discovered a new form of telomerase in the Ustilago maydis. Many dishes have a delicious effect on the taste of corn smut, for example tamales and taco. There are opportunities for finding novel mechanisms for the metabolism of genes in the corn smut.
Why do you want to study telomeraseRNA?
The discovery of how chromosomes are protected by telomeres was the reason for the prize. Telomerase was isolated from a unicellular organisms. telomerase plays a crucial role in aging and cancer and is found in almost all organisms. Scientists are trying to find a way to use telomerase to make human cells.
Human cells can't always be renewed. Human cells have a limited replicative life span, with older cells reaching this limit earlier than younger cells. The number of unique DNA repeats found at the ends of the genetic material-bearing chromosomes is 888-609- 888-609- 888-609- 888-609- 888-609- The ends of chromosomes are protected from unwanted and unwarranted DNA rearrangements by the protective capping structures.
The telomeric DNA will eventually fail to secure the chromosomes when the cell splits. A "molecular clock" counts down to the end of cell growth when the length of the telomeres is reduced continuously.
The diminished ability for cells to grow is related to the aging process and can lead to weakness, illness and organ failure.
The telomerase is the key to reversing the cellular aging process. Adding back lost DNA repeats to the clock helps extend the life span of the cell.
Telomerase shortens the length of the telomeres by repeating the sequence "GGTTAG" on the end of the chromosomes.
The replicative capacity of human stem cells is affected by the gradual shrinking of the telomeres. telomerase activity in adult stem cells slows down the clock on the molecule, but it does not cause the cells to be fully immortalized. Stem cells become exhausted in old people due to the shortening of the telomeres which results in increased healing times and organ tissue degradation.
Telomerase's full potential is being tapped.
Understanding the regulation and limitation of the telomerase is believed to hold the promise of reversing cellular aging with the potential to extend human life span and improve the wellbeing of elderly people.
Human diseases such as dyskeratosis congenita and aplastic anemia have been linked to genetic alterations that affect telomerase activity and/or shorten the lifespan. The shortened patient life span is caused by critically insufficient stem cell populations. The most promising way to treat these genetic diseases is by increasing telomerase activity.
Increased telomerase activity can bring youth to aging cells and cure premature aging-like diseases, but too much of a good thing can be harmful. Telomerase is used by cancer cells to maintain their growth. It will have to be done with precision, walking a narrow line between cell rejuvenation and a heightened risk for cancer.
The majority of the cells in the human body are not stem cells. The telomerase deficiency of human cells reduces their risk of developing cancer. Drugs that increase telomerase activityindiscriminately in all cell types are not desirable. Small molecule drugs can be used to increase telomerase activity in stem cells, without increasing the risk of cancer.
New mechanisms for telomerase regulation may be uncovered by the study of telomeraseRNA biogenesis in corn smut.
The study was published in theProceedings of the National Academy of Sciences. The team includes a first-time author and a former research assistant professor.
The caliber of the undergrad students who worked in his lab for over a year was commented upon by Chen. They spent a lot of time in the lab with us.
More information: Logeswaran, Dhenugen et al, Biogenesis of telomerase RNA from a protein-coding mRNA precursor, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2204636119. doi.org/10.1073/pnas.2204636119 Journal information: Proceedings of the National Academy of Sciences