A chemical in the cells of marine organisms allows them to survive in the deep oceans.
The pressure in the Mariana Trench is eight tons per square inch. The pressure at the Earth's surface increased by 1,100-fold.
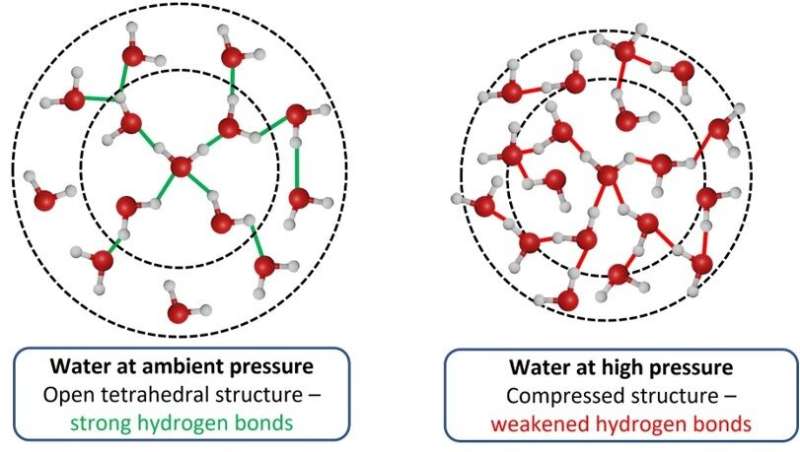
Water molecule form a network under pressure.
There is a network of water molecule.
The network of water molecule starts to change shape at high pressure. The water inside living cells prevents vital bio-chemical processes from taking place.
The first explanation of how a molecule found in the cells of marine organisms counteracts the effect of external pressure has been provided by the researchers.
Professor Dougan said that life has adapted to survive in the environment. Humans live in the depths of the oceans, where organisms live under high pressures.
The biomolecules that underpin all biological processes are adversely impacted by these high pressures.
Understanding what happens to water under pressure is important. We can apply these findings to the study of biomolecular stability if we understand how these organisms survive.
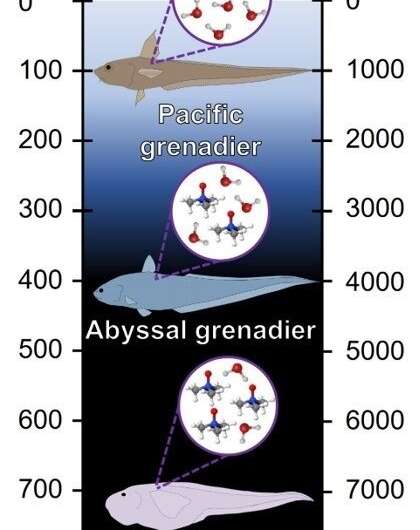
There is a compound called trimethylamine N-oxide.
There is a molecule found in cells that protects them against high external pressures. According to studies, the amount of TMAO increases in line with the depth of the ocean.
One of the most advanced analytical facilities in the world was used to conduct the study.
There is a scattering of neutraceutical particles.
The analytical facility at the STFC Rutherford Appleton Laboratory in Oxford was used to fire a beam of neutrons, which are sub-atomic particles. The analysis was done at low pressure and high pressure.
The atomic structure of the water molecule was revealed.
The hydrogen bonds in the pure water sample became less stable and the network of water molecule became smaller.
The network structure of the water molecule was maintained thanks to the presence of TMAO.
The water is able to resist the extreme pressure it is under thanks to the structural anchor provided by the TMAO. The findings help scientists understand how organisms have adapted to survive in the ocean.
The research team was able to develop an osmolyte protection ratio, which predicts the level of TMAO needed in the cells of marine organisms so they can survive at a specific depth in the ocean.
Professor Dougan said that the study provided a bridge between water under pressure at the molecule level and the wonderful ability of organisms which thrive under high pressure in depths of the oceans.
New species have been discovered at the bottom of the deep sea. Life has been able to exploit these habitats thanks to remarkable adaptation.
The paper "The ability of trimethylamine N-oxide to resist pressure induced perturbations to water structure" was published in the journal Communications chemistry.
More information: The ability of trimethylamine N-oxide to resist pressure induced perturbations to water structure, Communications Chemistry (2022). DOI: 10.1038/s42004-022-00726-z , www.nature.com/articles/s42004-022-00726-z.