The second most important starting product in the field of engineering is Propylene, a gas used to make a wide range of packaging and containers. It's production from propane is very energy intensive. The process accumulates side products that need to be burned frequently. It is very important to find a new approach to the production of this valuable molecule that is more efficient, produces fewer side products, and still uses materials that are stable at high temperatures.
A new catalyst developed by Hokkaido University material chemist Shinya Furukawa and his team can open up previously unavailable reaction pathways. Carbon dioxide can be used to turn propane into propylene, instead of Oxygen. The side effect of turning carbon dioxide into carbon monoxide, which is a useful resource for the production of many bulk chemicals, was demonstrated in the paper.
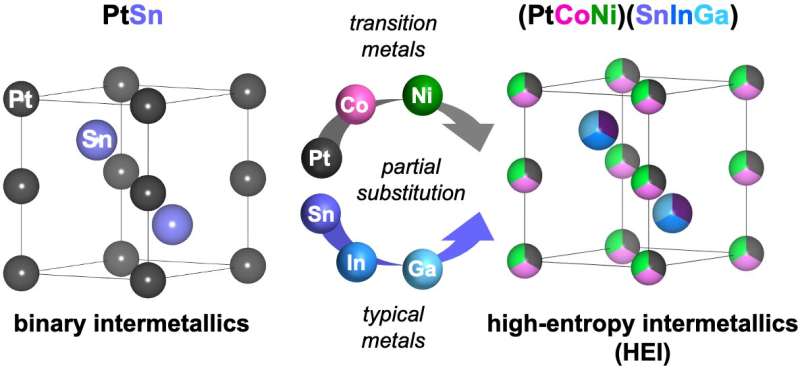
The researchers achieved this feat by building on their previous studies on catalyst design, but this time chose a unique new way: Using an alloy of Platinum and Tin on a ceria support as the base, they replaced a fraction of these atoms with the metals. Platinum-tin alloys had already been known as good catalysts for the reaction, but nickel and cobalt increased both the catalyst's ability to activated carbon dioxide and its selectivity to the desired reaction The catalyst's temperature stability was helped by the addition of indium and gallium. The support of ceria made it easier to remove carbon dioxide from the air. The research team confirmed that the catalyst can be regenerated and used again.
Furukawa says that this work shows the outstanding performance of our catalyst, but it also opens up a new window of catalyst design concepts based on our technique. Our previous Pt- Co-In catalyst was not as good as the new one. The carbon neutralization of the industrial production of small chemicals will be helped by these insights.
More information: Feilong Xing et al, High-entropy intermetallics on ceria as efficient catalysts for the oxidative dehydrogenation of propane using CO2, Nature Communications (2022). DOI: 10.1038/s41467-022-32842-8 Journal information: Nature Communications