The discovery of a peculiar structure and the quest to confirm it has led to the description of interactingreceptor clusters on natural killer cells. The study by the research team of Dr. Ondej Vank was published in the journal Nature Communications.
Dr. Ondej Vank is the leader of the laboratory. It all began a few years ago with an unexpected structure of a receptor and ended with a detailed description of the immune system.
Dr. Vank is interested in how the immune system cells distinguish between healthy and sick cells. His research team focuses on NK cells, which are part of innate immunity, and if they sense that another cell in the body is not healthy, can quickly remove it. Structural immunology seeks to understand how the immune cells recognize the other cell's structure. The NK cell knows whether or not all is okay. Dr. Vank says that the interplay of a number of interactions is what leads to the outcome.
The study focuses on two genes. NKR-P1 is areceptor onNK cells. The structure of this receptor is unknown, but it is interesting because it is one of the main surface markers. The NKR-P1 is found on the surface of some T lymphocytes which are implicated in several autoimmune diseases. In this context, however, its action is not yet well characterized and is likely to contribute to the development of these diseases.
The ligand of the NKR-P1receptor is the second part of the study. When cells interact and touch each other's surface, it makes them say they know about each other. The last fifteen years of research have shown that in many cases of cancer the LLT1 is expressed on the surface of the cancer cells. Dr. Vank said, "Unfortunately, the worse the tumor type, the higher surface expression of LLT1Protein." The structure of LLT1 was described by him and his colleagues.
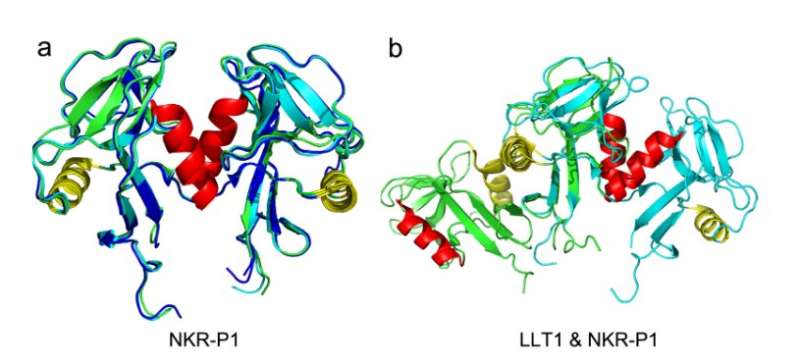
The atomic structure to the cellular level is described in the paper. The structure of the complex was solved by the research team.
The result was rather surprising. Dr. Vank wondered if this was just an artifact of the crystal or a structure on the cell surface. The next phase of the study was carried out on the cell surface and live cells isolated from donor blood. The research team verified previous observations in the crystal structure of the complex and described the resulting functional consequences under certain conditions.
Two identical chains on the cell surface are connected by disulfide bonds by the NKR-P1receptor and LLT1receptor. The idea so far has been that when the two proteins interact, one of them will bind one of the other's dimers. Thanks to the crystal structure of the NKR-P1 complex, we know that this is not the case.
The hypothesis was tested from the atomic level to the cellular level. The affinity of the studied proteins is very weak and only through clustering can it become strong enough for the cell to sense the signal. Thanks to the new study, we can see how this interaction works at the structural level. The interaction between the immune system and cancer cells might be influenced by this.
The study was carried out by Dr. Ondej Vank and his team at the Faculty of Science of Charles University. Two researchers from the University of Oxford made significant contributions to the research.
Several generations of students from our lab have been involved in this study and the first author did his PhD on this research." Students advanced a lot as we learned more and more methods. Dr. Vank says that some of them are working at top European research institutions.
The most interesting thing for me while working on this project was discovering new insights in relatively common data that led to more complex experiments. I didn't have to be afraid of following my own crazy ideas if they are based on the data. Many of the world's experts are only humans and the most passionate ones are willing to help with crazy scientific ideas.
More information: Jan Bláha et al, Structure of the human NK cell NKR-P1:LLT1 receptor:ligand complex reveals clustering in the immune synapse, Nature Communications (2022). DOI: 10.1038/s41467-022-32577-6 Journal information: Nature Communications